
Serum vitamin D status among healthy children in Hainan, South China: a multi-center analysis of 10,262 children
Introduction
Vitamin D (VD) plays an important role in calcium/phosphorus metabolism, bone health, and a variety of other biological functions (1). Besides the traditional vitamin D deficiency (VDD) associated rickets and tetany, VDD or vitamin D insufficiency (VDI) is also closely related to many non-skeletal diseases, including neuropsychological diseases (such as autism, schizophrenia, and depression), cardiovascular and metabolic diseases (including obesity, diabetes, and hypertension), rheumatoid immune diseases (such as rheumatoid arthritis and systemic lupus erythematosus), infectious diseases (including pneumonia and tuberculosis), and even certain types of cancers (2-10). Studying vitamin D status in children aged ≤18 can help guide children’s reasonable vitamin D supplementation, which is very important for children’s health.
Global epidemiological surveys showed that the prevalence of VDD in European countries is up to 40.4% (11). The VD levels in Asian countries are highly variable. For example, the prevalence of VDD among children in southern India is about 30%, while that in northern India is as high as 50%. In Japan, 7.2% of the infants younger than 48 months have VDD and 13.8% present with VDI (12). Among American countries, the prevalence of VDD and VDI among children in the United States is 9.7% and 56%, respectively. In Canada, the prevalence of VDD is 6%, and that of VDI is 24% (13). In Southern Tasmania, 8% of 8-year-old children and 68% of 16-year-old children have VDD (14). The prevalence of VDD among African children in Ethiopian is up to 42% (15). In China, Wu et al. measured the serum 25-hydroxyvitamin D (25-OHD) levels of 222 healthy adolescents (12–15 years old) in Beijing, China and reported that the prevalence of VDD was up to 97% (16), which is much higher than that in other cities. Xu et al. (17) found that, in Hong Kong between 2009 and 2010, children aged 6–17 years had significantly lower serum 25-OHD levels compared to adults. Ke et al. reported that Chinese people, aged 18–93 years old, living in Macau had a VDD prevalence of 55% (18) and the prevalence of VDD in pregnant women in Wuxi City, China (latitude: 31.50 N) was 38.0% (19).
Hainan is the southernmost province in China and is also the only province that completely lies in a tropical region (latitude: 3.30–20.17 N). Hainan has a maritime tropical monsoon climate, with plenty of sunshine throughout the year. Moreover, the daily diet of the Hainanese includes many marine products (such as fish), which are rich in VD. However, there is still a paucity of data related to VD levels in large populations of Chinese children, especially for such a uniquely located province in China. This current multicenter study evaluated the VD status of children in Hainan using a large sample size, so as to provide scientific data for the prevention and treatment of children with VDD or VDI in this region. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-235/rc).
Methods
Study subject
A total of 10,262 healthy children who underwent health examinations at four hospitals in Hainan (including Hainan Women and Children’s Medical Center, Hainan General Hospital, Haikou Maternity and Child Health Hospital, and Sanya Maternity and Child Health Hospital) from 2012 to 2020 were included in this study. Children with abnormal renal function or other metabolic diseases that may interfere with VD metabolism were excluded. The age range of the included children was 0.09–17.00 years old, with an average age of 3.32±2.96 years. Children were divided into five age groups: 0–1, 1–3, 3–7, 7–14, and 14–18 years. There were 2,093 cases in the 0–1 year group, 4,242 cases in the 1–3 years old group, 2,707 cases in the 3–7 years group, 1,184 cases in the 7–14 years old group, and 36 cases in the 14–18 years group. There were 5,861 boys and 4,401 girls. Based on the time of examination, the children were divided into 4 groups: spring (from February to April, 2086 cases), summer (from May to July, 2,877 cases), autumn (from August to October, 2917 cases), and winter (from November to January, 2,382 cases). According to the growth curve of the body mass index (BMI) of children aged 0 to 18 years old in China (20), children with BMI ≥95th percentile of normal children with the same age range and sex were defined as obese, and those with BMI between the 85th and 95th percentile were defined as overweight. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Hainan Women and Children’s Medical Center (HNWCMC Ethical Approval 2011[01]). Hainan General Hospital, Haikou Maternity and Child Health Hospital, and Sanya Maternity and Child Health Hospital were informed and agreed with this study. The legal guardians of enrolled children signed informed consent forms.
Detection methods and diagnostic criteria
Venous blood samples were collected from children in the early morning before meals. The samples were sent to the laboratory for testing within 2 h of collection. The serum 25-OHD levels were measured using a Roche automatic chemiluminescence detector. According to the International Classification of Children’s VD Nutritional Status, the results were classified using the seven-category method or the three-category method. According to the seven-category method, 25-OHD levels <12.5 nmol/L is considered severe deficiency, 12.5–25 nmol/L is moderate deficiency, 25–50 nmol/L is mild deficiency, 50–75 nmol/L is VDI, 75–250 nmol/L is normal, 250–375 nmol/L is excessive, and ≥375 nmol/L is VD poisoning. According to the three-category method, 25-OHD levels <50 nmol/L is considered VDD, 50–75 nmol/L is VDI, and ≥75 nmol/L is VD sufficient (VDS).
Statistical methods
Statistical analysis was performed using SPSS 20.0 software. Analyses of the data for the serum 25-OHD concentrations revealed a normal distribution. The data were therefore described using mean ± standard deviation. The t-test was used to compare differences of VD levels between groups. The nonparametric chi-square test was used to compare different rates between groups. Logistic regression analysis was used to obtain odds ratio in the presence of possible factors and estimate partial correlation coefficients. A two-sided P value of 0.05 was considered to be statistically significant.
Results
The overall levels of serum 25-OHD in the test population
The serum 25-OHD levels of 10,262 children showed a normal distribution, with an average of 94.63±49.99 nmol/L [95% confidence interval (CI): 93.67–95.60] and a range of 50–90 nmol/L (Figure 1). The average serum 25-OHD levels of the 5,861 boys was significantly higher than that of the 4,401 girls (96.21±51.27 and 92.55±48.16 nmol/L, respectively; t=3.67, P<0.001).
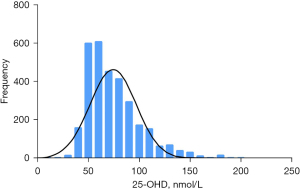
Among the participating children, 14% had VDD (1,435/10,262), including 2 cases of severe deficiency, 49 cases of moderate deficiency, and 1,384 cases of mild deficiency. VDI was detected in 30.6% of participants (3,140/10,262) and VDS was noted in 55% of the study population (5,687/10,262), including 5,583 cases with acceptable VD levels and 104 cases with VD overdose. There was no cases of VD poisoning. Therefore, according to the seven-category method, 54.4% children (5,583/10,262) had an acceptable VD level (50–75 nmol/L), 30.6% children (3,140/10,262) were VDI (50–75 nmol/L), 13.5% children (1,384/10,262) were mild deficiency (25–50 nmol/L), last 1.5% (155/10,262) contained 104 cases VD overdose (250–375 nmol/L), 49 cases moderate deficiency (12.5–25 nmol/L) and 2 cases sever deficiency (<12.5 nmol/L) (Figure 2). There were 1,590 children with complete height and weight data, including 1,442 cases in the normal group, 64 cases in the overweight group, and 84 cases in the obese group.
A comparison of the serum 25-OHD levels and the VD nutritional status in different age groups
The 25-OHD levels and VD nutritional status varied in different age groups of children (Table 1). The highest average 25-OHD levels were found in the 0–1 year old group (105.92±57.39 nmol/L), while children aged 14–18 years old had the lowest 25-OHD levels (54.97±19.19 nmol/L). As the age increased, the 25-OHD levels showed a downward trend, and the difference among different age groups was statistically significant (F=123.78, P<0.001). Among the 5 age groups, children aged 14–18 years had the highest VDD and VDI ratios (VDD accounted for 38.9% and VDI accounted for 44.4%), and VDS only accounted for 16.7%. Children aged 0–1 and 1–3 years had a higher prevalence of VDS, at 60% and 59.2%, respectively. The distribution of VD nutritional status was significantly different among all age groups (χ2=237.134, P<0.001).
Table 1
| Parameters | No. of cases | 25-OHD (nmol/L) | VD nutritional status, n (%) | ||||
|---|---|---|---|---|---|---|---|
| 95% CI | VDD | VDI | VDS | ||||
| Age group (years) | |||||||
| 0–1 | 2,093 | 105.92±57.39 | 103.46–108.39 | 289 (13.8) | 549 (26.2) | 1,255 (60.0) | |
| 1–3 | 4,242 | 100.55±53.22 | 98.95–102.15 | 542 (12.8) | 1,190 (28.1) | 2,510 (59.2) | |
| 3–7 | 2,707 | 86.35±39.19 | 84.88–87.84 | 346 (12.8) | 888 (32.8) | 1,473 (54.4) | |
| 7–14 | 1,184 | 73.61±34.21 | 71.66–75.56 | 244 (20.6) | 497 (42.0) | 443 (37.4) | |
| 14–18 | 36 | 54.97±19.19 | 48.48–61.47 | 14 (38.9) | 16 (44.4) | 6 (16.7) | |
| Total | 10,262 | 94.63±49.99 | 1,435 (14.0) | 3,140 (30.6) | 5,687 (55.4) | ||
| F/χ2 | 123.78 | 237.134 | |||||
| P | <0.001 | <0.001 | |||||
25-OHD, 25-hydroxyvitamin D; VD, vitamin D; CI, confidence interval; VDD, vitamin D deficiency; VDI, vitamin D insufficiency; VDS, vitamin D sufficiency.
A comparison of serum 25-OHD levels in different seasons
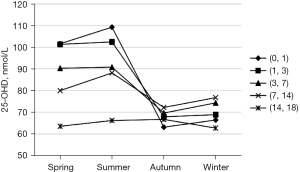
The serum 25-OHD levels of children varied according to different seasons (Table 2). The average 25-OHD levels in spring and summer were relatively high (96.56±45.76 and 96.85±52.23 nmol/L, respectively), declining in autumn (94.53±50.40 nmol/L), and reaching the lowest average levels in winter (90.41±50.03 nmol/L). The difference in the 25-OHD levels among different seasons was statistically significant (F=8.606, P<0.01). Moreover, the seasonal changes of serum 25-OHD levels were similar in different age groups (Figure 3).
Table 2
| Parameters | Number of cases |
25-OHD (nmol/L) | |
|---|---|---|---|
| 95% CI | |||
| Season | |||
| Spring | 2,086 | 96.56±45.76 | 94.60–98.53 |
| Summer | 2,877 | 96.85±52.23 | 94.94–98.76 |
| Autumn | 2,917 | 94.53±50.40 | 92.70–96.36 |
| Winter | 2,382 | 90.41±50.03 | 88.40–92.42 |
| Total | 10,262 | 94.64±49.99 | |
| F | 8.606 | ||
| P | <0.01 | ||
25-OHD, 25-hydroxyvitamin D.
A comparison of the serum 25-OHD levels and VD nutritional status in children with different body weights
In this study cohort, 1,590 children had valid height and weight data (Table 3). Children with normal weight (BMI <85th percentile) had higher 25-OHD levels (115.72±31.62 nmol/L) compared to the obese group (BMI ≥95th percentile) and the overweight group (BMI ≥85th percentile). The average 25-OHD levels of the overweight group was the lowest (103.13±36.20 nmol/L). There was a significant difference in 25-OHD levels among these three groups (F=7.393, P=0.001). For VD nutritional status, children with BMI <85th percentile had the lowest VDD ratio (0.83%) and the highest VDS ratio (91.82%); while overweight children had the highest VDD ratio (6.25%), and obese children had the highest VDI ratio (19.05%). The difference in VD nutritional status among the three groups was statistically significant (χ2=37.177, P<0.001).
Table 3
| Parameters | No. of cases | 25-OHD (nmol/L) | VD nutritional status, n (%) | ||||
|---|---|---|---|---|---|---|---|
| 95% CI | VDD | VDI | VDS | ||||
| BMI group | |||||||
| <85th percentile | 1,442 | 115.72±31.62 | 114.08–117.35 | 12 (0.83) | 106 (7.35) | 1,324 (91.82) | |
| ≥85th percentile | 64 | 103.13±36.20 | 94.08–112.17 | 4 (6.25) | 10 (15.63) | 50 (78.12) | |
| ≥95th percentile | 84 | 106.94±34.29 | 99.50–114.38 | 2 (2.38) | 16 (19.05) | 66 (78.57) | |
| Total | 1,590 | 114.74±32.09 | 113.16–116.32 | 18 (1.13) | 132 (8.30) | 1440 (90.57) | |
| F/χ2 | 7.393 | 37.177 | |||||
| P | 0.001 | <0.001 | |||||
25-OHD, 25-hydroxyvitamin D; BMI, body mass index; VD, vitamin D; CI, confidence interval; VDD, vitamin D deficiency; VDI, vitamin D insufficiency; VDS, vitamin D sufficiency.
Logistic regression analysis of the factors affecting 25-OHD levels in children
A backward (conditional) logistic regression analysis was performed where VD sufficiency was used as the independent variable Y (0 is insufficient, where 25-OHD <75 nmol/L and 1 is sufficient, where 25-OHD ≥75 nmol/L). The dependent variables were gender (X1, where 1 is male and 2 is female), age (X2, where 1–5 represent the different age groups), and season (X3, where 1–12 represent the 12 months of the year). The results indicated that gender, age, and season all had a certain impact on VD sufficiency (Table 4).
Table 4
| Factors | B | SE | Wald (χ2) | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Gender | −0.096 | 0.041 | 5.565 | 0.018 | 0.909 | 0.839–0.984 |
| Age | −0.266 | 0.022 | 150.715 | <0.001 | 0.767 | 0.735–0.800 |
| Season | −0.038 | 0.006 | 35.488 | <0.001 | 0.963 | 0.951–0.975 |
SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
This study analyzed the distribution of serum 25-OHD levels in 10,262 children in the Hainan province. Children with VD insufficiency (serum 25-OHD <75 nmol/L) accounted for 44.58%, which is nearly half of the study population. Interestingly, the serum 25-OHD levels of children in Hainan was higher than that reported in Beijing (16). The overall levels of VD varied greatly between the northern and southern cities, and this may be related to diet and living habits, regional latitude and altitude, sunshine exposure time, awareness of VD insufficiency and associated preventive measures, as well as the general knowledge of the population (21).
There were 104 cases (1.01%) with VD overdose. A review of the outpatient data and follow-up record revealed that most of the VD overdose cases had a history of single or multiple high-dose VD intake, and their 25-OHD levels had not been regularly monitored. This is consistent with the common causes of VD overdose reported in other regions (22,23). Further stratifying the VD overdose group by age demonstrated that, among the children with VD overdose, 39 cases (37.5%) were in 0–1 year group, 55 cases (52.9%) were in 1–3 years group, 8 cases (7.7%) were in 3–7 years group, and 2 cases (1.9%) were in 7–14 years group. The children under 3 years accounted for 90.4% of the VD overdose cases, which may be related to parents focusing on VD supplementation for infants but overlooking VD monitoring. Therefore, it is important to pay attention to the monitoring of VD levels in children under 3 years of age.
This study found that gender, age, and season all had a certain impact on VD sufficiency, which is consistent with other reports (24,25). The overall serum VD levels of boys was significantly higher than that of girls, which may be related to more outdoor activities, less physical sun protection measures, longer exposure to sunlight, different hormone levels, and different dietary structures of boys. In addition, VD levels were negatively correlated with age, which may be related to dietary factors such as the discontinuation of VD supplementation after 3 years of age and reduction in dairy product intake, environmental factors such as reduced outdoor activities and reduced exposure to sunshine, and social factors such as reduced concern from parents about the VD nutrition status after school age. The serum VD levels of children under 3 years old was significantly higher than that of other age groups, which may be related to the VD from the mother’s milk, and the continuous supplementation of VD 400–800 IU/d as recommended in the “Recommendations for the Prevention and Treatment of Rickets” in China (mainly for infants and young children) (26). Therefore, it should be recommended to systematically manage the VD nutritional status of children after 3 years of age. The seasonal analysis revealed that the 25-OHD levels in winter were significantly lower than those in the other three seasons, which is consistent with the annual radiation change in Hainan, in which March, July, and October experience peak sunshine levels, while December is the winter-spring transition, when the cold air frequently goes southward, leading to thicker clouds and shorter sunshine hours (27).
There were some limitations to this study. This investigation was unable to retrospectively collect height and weight data for all the children. Only 1,590 children had complete height and weight data, which were used to analyze the relationship between BMI and VD nutritional status. This study did not collate other parameters such as VD supplementation status, dairy product consumption, outdoor activity time, and the guardian’s educational level and economic situation. Thus, it is unclear whether other factors may affect VD levels. In addition, the sample size in this study was large and the time span was also long. Therefore, the economic level, living habits, and nutritional status of residents may change during the course of the study. These factors may have affected the study results.
In summary, this report found that healthy children of all ages in Hainan showed a certain percentage of VDD and VDI, especially in those over 7 years old. In 2011, the American Endocrine Society recommended that the amount of VD supplementation should be 400–1,000 U/d for infants and 600–1,000 U/d for children aged 1–18 years old. The “Recommendations for the Prevention and Treatment of Rickets” of China recommends that the prevention of VDD should be continued from the perinatal period to adolescence, and should be “adjusted according to time, place, and person” (26). Therefore, in addition to sunbathing and dietary intake, VD supplementation should be considered, especially for children over 3 years old. Moreover, regular monitoring of VD levels is required during VD supplementation to avoid VD overdose.
Acknowledgments
We would like to thank professor Xiao Le from School of Pediatrics, Hainan Medical University provided kind help in paper writing.
Funding: This study was supported by the Hainan Major Science and Technology Projects (Nos. ZDKJ2019010 and HXK200020), the Excellent Talent Team of Hainan Province (No. QRCBT202121) and the Hainan Province Clinical Medical Center (No. QWYH202175).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-235/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-235/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-235/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Hainan Women and Children’s Medical Center (HNWCMC Ethical Approval 2011[01]). Hainan General Hospital, Haikou Maternity and Child Health Hospital, and Sanya Maternity and Child Health Hospital were informed and agreed with this study. The legal guardians of enrolled children signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Michigami T. Rickets/Osteomalacia. Consensus on Vitamin D Deficiency and Insufficiency in Children. Clin Calcium 2018;28:1307-11. [PubMed]
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080S-6S. [Crossref] [PubMed]
- Beydoun MA, Hossain S, Fanelli-Kuczmarski MT, et al. Vitamin D Status and Intakes and Their Association With Cognitive Trajectory in a Longitudinal Study of Urban Adults. J Clin Endocrinol Metab 2018;103:1654-68. [Crossref] [PubMed]
- Gallardo-Carrasco MC, Jiménez-Barbero JA, Bravo-Pastor MDM, et al. Serum Vitamin D, Folate and Fatty Acid Levels in Children with Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J Autism Dev Disord 2021; Epub ahead of print. [Crossref] [PubMed]
- Zhang P, Guo D, Xu B, et al. Association of Serum 25-Hydroxyvitamin D With Cardiovascular Outcomes and All-Cause Mortality in Individuals With Prediabetes and Diabetes: Results From the UK Biobank Prospective Cohort Study. Diabetes Care 2022;45:1219-29. [Crossref] [PubMed]
- Alagacone S, Verga E, Verdolini R, et al. The association between vitamin D deficiency and the risk of resistant hypertension. Clin Exp Hypertens 2020;42:177-80. [Crossref] [PubMed]
- Ao T, Kikuta J, Ishii M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021;11:1624. [Crossref] [PubMed]
- Papagni R, Pellegrino C, Di Gennaro F, et al. Impact of Vitamin D in Prophylaxis and Treatment in Tuberculosis Patients. Int J Mol Sci 2022;23:3860. [Crossref] [PubMed]
- Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018;50:1-14. [Crossref] [PubMed]
- Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol 2018;175:177-89. [Crossref] [PubMed]
- Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 2016;103:1033-44. [Crossref] [PubMed]
- Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol 2007;103:620-5. [Crossref] [PubMed]
- Kumar J, Muntner P, Kaskel FJ, et al. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics 2009;124:e362-70. [Crossref] [PubMed]
- Jones G, Dwyer T, Hynes KL, et al. Vitamin D insufficiency in adolescent males in Southern Tasmania: prevalence, determinants, and relationship to bone turnover markers. Osteoporos Int 2005;16:636-41. [Crossref] [PubMed]
- Wakayo T, Belachew T, Vatanparast H, et al. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: the case of school children in central Ethiopia. PLoS One 2015;10:e0120963. [Crossref] [PubMed]
- Wu F, Laslett LL, Zhang Q. Threshold Effects of Vitamin D Status on Bone Health in Chinese Adolescents With Low Calcium Intake. J Clin Endocrinol Metab 2015;100:4481-9. [Crossref] [PubMed]
- Xu C, Perera RA, Chan YH, et al. Determinants of serum 25-hydroxyvitamin D in Hong Kong. Br J Nutr 2015;114:144-51. [Crossref] [PubMed]
- Ke L, Mason RS, Mpofu E, et al. Vitamin D and parathyroid hormone status in a representative population living in Macau, China. J Steroid Biochem Mol Biol 2015;148:261-8. [Crossref] [PubMed]
- Xiao JP, Zang J, Pei JJ, et al. Low maternal vitamin D status during the second trimester of pregnancy: a cross-sectional study in Wuxi, China. PLoS One 2015;10:e0117748. [Crossref] [PubMed]
- Li H, Ji CY, Zong XN, et al. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi 2009;47:493-8. [PubMed]
- Tolppanen AM, Fraser A, Fraser WD, et al. Risk factors for variation in 25-hydroxyvitamin D3 and D2 concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab 2012;97:1202-10. [Crossref] [PubMed]
- Galior K, Grebe S, Singh R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018;10:953. [Crossref] [PubMed]
- Ketha H, Wadams H, Lteif A, et al. Iatrogenic vitamin D toxicity in an infant--a case report and review of literature. J Steroid Biochem Mol Biol 2015;148:14-8. [Crossref] [PubMed]
- Karacan M, Usta A, Biçer S, et al. Serum vitamin D levels in healthy urban population at reproductive age: effects of age, gender and season. Cent Eur J Public Health 2020;28:306-12. [Crossref] [PubMed]
- Wang LK, Hung KC, Lin YT, et al. Age, Gender and Season Are Good Predictors of Vitamin D Status Independent of Body Mass Index in Office Workers in a Subtropical Region. Nutrients 2020;12:2719. [Crossref] [PubMed]
- Yang SF, Wu GC. Recommendations for the prevention of Vitamin D deficiency and rickets. Chinese Journal of Child Health Care 2015;23:781-2.
- Peng YS, Wang Y, Lan Q. The climatologically calculauion and distributive characteristics of global solar radiation in Hainan Province. Natural Science Journal of Hainan University 2007;25:259-61.



