
Systematic review and meta-analysis of the incidence rates of adverse events after digestive endoscopy in children
Introduction
Digestive endoscopy is the most common and reliable approach for digestive tract diseases (1). Since the first research report in 1963 regarding gastroscopy in children, gastrointestinal endoscopy has been gradually introduced into the diagnosis and therapy of various pediatric diseases, and has played a substantial role in treating pediatric digestive system diseases (2). Common pediatric endoscopy examinations include gastroscopy, colonoscopy, capsule endoscopy (CE), endoscopic ultrasonography (EUS), and endoscopic retrograde cholangiopancreatography (ERCP) (3). Among them, gastroscopy can significantly improve the etiological diagnosis rate of upper gastrointestinal bleeding and the detection rate of acute gastric mucosal lesions within 48 hours (4). The detection rate of repeated gastrointestinal bleeding, iron deficiency anemia, or chronic diarrhea by double-balloon enteroscopy under general anesthesia was as high as 85.7% (5). Enteroscopic surgical resection significantly reduced the rate of emergency surgery in children with polyp syndrome (6). New colon capsule endoscopy (CCE) achieved sensitivity and specificity of 96% and 100% in monitoring and evaluating ulcerative colitis in children, respectively (7). EUS is an effective means for diagnosing perianal rectal abnormalities, congenital muscular hypertrophy, lymphoma, and polyposis in children (8).
With the widespread application of digestive endoscopy in children, adverse events (AEs) such as myoclonus, abdominal pain, fever, bleeding, chest pain, sore throat, vomiting, anosensation, esophageal reflux or ulcer, rash, delayed capsule discharge, perforation, pancreaticobile duct infection, and pancreatitis have occurred in children after digestive endoscopy (9,10). Statistical results indicated that the AEs after painless gastroenteroscopy reached 26.98% incidence (11). After CE, 1.5% to 3.5% of children were trapped in digestive tract for more than 2 weeks, accompanied by nausea and vomiting (12). After ERCP examination, patients were accompanied by adverse reactions and complications such as drug reaction, infection (cholangitis, etc.), perforation, and pancreatitis, and the incidence of adverse reactions was 6% (13). The incidence of perforation during colonoscopy in children was 0.01%, of which 0.2–2.5% were bleeding perforation after biopsy (14). The incidence of perforation by colonoscopy was 0.96% (15). At the same time, some studies showed that the incidence of grade 3 or higher bleeding (requiring hospitalization and intervention, such as blood transfusion or repeat endoscopy) whose incidence rate was 0.11% among all children undergoing endoscopy (16). Due to the differences in sample size, study method, study population, observation time, and other factors, there are significant differences in the statistical results of AEs after digestive endoscopy in children, which is difficult to reflect the actual level and severity of AEs after digestive endoscopy in children.
To systematically evaluate the adverse reaction incidence in children with digestive endoscopy, the incidence of AEs after gastrointestinal endoscopy in children was collected, summarized, and analyzed by meta-analysis, and the incidence and safety of AEs in gastrointestinal diseases in children were evaluated, so as to provide medical evidence for the diagnosis and treatment of gastrointestinal diseases in children. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-179/rc).
Methods
Methods of including data
Children who underwent gastroscopy, colonoscopy, CE, EUS, ERCP, and other digestive endoscopy were recruited as participants. The studies included were retrospective and prospective. The data collection included the author, year, country, sample size of participants, age of participants, and outcome indicators.
Inclusion and exclusion criteria
Articles which met the below requirements were eligible for inclusion in this meta-analysis: (I) literature published from January 2005 and October 2021; (II) participant cohort comprised more than 1 child who underwent digestive endoscopy; (III) the articles analyzed any AE caused by digestive endoscopy; (IV) the research types were cross-sectional study, case-control study, and cohort study; and (V) the articles recorded baseline indicators such as age, gender, source of cases, and the occurrence of AEs caused by digestive endoscopy was also recorded and counted in detail.
Articles which met the below conditions were excluded: (I) articles were publicity literature such as individual case reports, literature reviews, expert comments, editorial opinions, news reports, and product descriptions; (II) no original data; (III) repeat publications; (IV) articles not related to AEs caused by digestive endoscopy; (VI) articles reported the number of AEs caused by digestive endoscopy but did not calculate the incidence of AEs; and (V) basic research articles such as animal experiments and in vitro cell experiments.
Retrieval strategy
The system retrieved relevant information contained in various online databases, and the retrieval time range of each database was from 2005 to October 2021. The PubMed (2005–October 2021), Nature (2005–October 2021), Web of Science (2005–October 2021), Spring (2005–October 2021), CNIKI (2005–October 2021), WanFang (2005–October 2021), and Science Direct (2005–October 2021) databases were searched with the keywords “capsule endoscopy”, “gastroscope”, “endoscopic ultrasonography”, “EUS”, “endoscopic retrograde cholangiopancreatography”, “ERCP”, “enteroscope”, “colonoscopy”, “colonoscopy enteroscope”, “children”, “adverse events”, and “adverse respiratory events”. The keywords were combined with “OR” or “AND”. We searched for clinical studies on AEs caused by digestive endoscopy in children published between 2005 and October 2021. All search keywords were freely combined. The search time range was set from 1 January 2005 to 1 October 2021. No language restrictions were required.
Literature screening and quality evaluation
Independently, the quality evaluation and data extraction were implemented by two reviewers, and the unqualified and low quality ones were excluded. In case of inconsistency in the results, the two reviewers decided upon inclusion of the article via discussion, or it was decided by a third party.
Eleven evaluation criteria recommended by the Agency for Healthcare Research and Quality (AHRQ) were adopted for risk of bias assessment, including the following contents: (I) whether the source of the data is clear; (II) whether the inclusion and exclusion criteria of exposed group and non-exposed group are listed; (III) whether the time period for identifying patients is given; (IV) if it is not from the population, whether the research object is continuous; (V) whether the subjective factors of the evaluator cover up other aspects of the research object; (VI) whether it describes any assessment to ensure quality; (VII) whether the reasons for excluding any patients are explained; (VIII) whether measures to evaluate and/or control confounding factors are described; (IX) whether it explains how to deal with lost data in analysis, if possible; (X) whether it summarizes the response rate of patients and the integrity of data collection; (XI) if there is follow-up, whether the number (ratio) of patients with incomplete data or follow-up results are described. The evaluation criteria were answered with “yes” (1 point), “no” (0 point), or “unclear” (0 point), and the quality score was given. The full score was 11 points, 0–3 was low quality, 4–7 was medium quality, and 8–11 was high quality.
Extraction of article data
Two literature reviewers finished the data extraction. First, basic data included title, first author, publication year, journal, research type, and research duration. Second, the number of participants, the age of the participants, and the type and number of AEs. Third, through literature search and expert consultation, AEs related to digestive endoscopy were identified: adverse respiratory events, myoclonus, abdominal pain, fever, bleeding, chest pain, sore throat, vomiting, anesthesia, esophageal reflux, rash, and delayed capsule expulsion.
Statistical methods
All data was sorted out using Excel 2016, and the standards of AHRQ Evidence-based Practice Center were adopted for quality assessment. RevMan5.3 and Stata 17.0 were employed for meta-analysis of the extracted data.
Methodological heterogeneity was determined by the differences between the results of different study design literature. Statistical heterogeneity was determined regarding Q test (P) and I2, and the significance level was set as α=0.05 and P<0.05. If I2<25%, there was low heterogeneity in the literature. If 25%<I2<50%, there was moderate heterogeneity. If I2>50%, there was substantial heterogeneity. When I2<50%, the fixed-effect model (FEM) was utilized for meta-analysis. When I2>50%, random effects model was employed. The incidence of each AE and 95% confidence interval (CI) were calculated. Meta-analysis was conducted by combining the rates of different types of AEs to obtain the combined results of the incidence of each AE and the combined results of the total incidence of AEs. Sensitivity was analyzed by eliminating the included literatures one by one to evaluate the stability of the meta-analysis results. Funnel plot and Egger’s test were adopted for publication bias evaluation. In addition, the meta-analysis results were indicated by Z value and P value, and each effect size was illustrated with a 95% CI. Bilateral test was performed at α=0.05. P<0.05 was deemed as significant difference.
Results
Article retrieval process
The databases of PubMed, Web of Science, Spring, Nature, and Science Direct were searched using the search terms “capsule endoscopy”, “gastroscope”, “endoscopic ultrasonography”, “EUS”, “endoscopic retrograde cholangiopancreatography”, “ERCP”, “enteroscope”, “colonoscopy”, “colonoscopy enteroscope”, “children”, “adverse events”, “adverse respiratory events”, and “meta-analysis”. In all, 1,292 works were obtained from databases, and 604 works were obtained from registers, the 183 articles were included after preliminary screening. Among them, 95 were from PubMed, 32 were from Web of Science, 24 articles were retrieved from the Spring database, 13 articles were retrieved from the Nature database, 19 articles were retrieved from Science Direct, and 10 were from the referenced literature and reviewed references. After articles that didn’t meet the inclusion criteria were eliminated, 53 were retained. Reviews, conference essays, case analyses, and risk factor assessments were excluded regarding their titles, article abstracts, and research contents. 22 articles were initially conformed to the inclusion criteria in preliminary screening. After further intensive screening, 7 articles for which original data and uncontrolled studies could not be obtained were excluded, and 15 articles were finally included. The whole process is shown in Figure 1.
Basic information
Of the 15 articles (17-31) that were finally included, 13 were Cross-sectional study, accounting for the highest proportion, including 27,770 children. The basic information is shown in Table 1.
Table 1
| The first author | Year of publication | Country | Type | Total sample size | AHRQ score |
|---|---|---|---|---|---|
| Biber (17) | 2015 | America | Cross-sectional study | 12,030 | 7 |
| Flores-González (18) | 2019 | Spain | Cross-sectional study | 88 | 5 |
| Gu (19) | 2019 | China | Cohort study | 129 | 5 |
| Iwama (20) | 2019 | Japan | Cross-sectional study | 183 | 6 |
| Kramer (21) | 2016 | America | Cross-sectional study | 9,577 | 8 |
| Lee (22) | 2020 | America | Cross-sectional study | 929 | 6 |
| Mamula (23) | 2005 | America | Cross-sectional study | 60 | 5 |
| Moy (24) | 2007 | America | Cross-sectional study | 45 | 5 |
| Najafi (25) | 2019 | Belgium | Cross-sectional study | 3,435 | 7 |
| Oliva (26) | 2016 | Italy | Cross-sectional study | 40 | 6 |
| Oliva (27) | 2014 | Italy | Cross-sectional study | 29 | 4 |
| Pai (28) | 2019 | America | Cross-sectional study | 98 | 7 |
| Reddy (29) | 2021 | India | Cross-sectional study | 189 | 6 |
| Sanders (30) | 2021 | America | Cross-sectional study | 122 | 4 |
| Wani (31) | 2020 | India | Case-control study | 822 | 5 |
AHRQ, Agency for Healthcare Research and Quality.
Quality evaluation
AHRQ criteria were adopted to assess the article quality. Among the 15 included literatures, 1 was 8 points of Joanna Briggs Institute (JBI), 3 were 7 points of JBI, and 4 were 6 points of JBI. There were 5 literatures with JBI score of 5 and two literatures with a JBI score of 4 (Table 1).
Meta-analysis of the incidence of adverse respiratory events
Of the 15 included articles, a total of 7 articles reported the incidence of adverse respiratory events after digestive endoscopy. As displayed in Figure 2, no significant heterogeneity was suggested in the incidence of AEs in different articles (I2=96%; P<0.00001), so the random-effect model (REM) was utilized. The risk ratio (RR) value of adverse respiratory events after digestive endoscopy was 1.31 (95% CI: 1.17 to 1.47), and notable heterogeneity was found in the incidence of adverse respiratory events after digestive endoscopy (Z=4.55; P<0.00001).
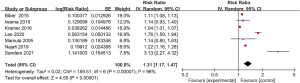
Meta-analysis of the incidence of myoclonus
The incidence of myoclonus in the 4 included articles was compared (Figure 3). The heterogeneity in the incidences of myoclonus after digestive endoscopy in children was first analyzed, and a high degree of heterogeneity was indicated (I2=89%; P<0.00001), so a REM was adopted. It turned out that the odds ratio (OR) value of myoclonus occurrence after digestive endoscopy in children was 1.21 (95% CI: 1.01 to 1.46), and there was heterogeneity in the incidence of myoclonus after digestive endoscopy in children (Z=2.04; P=0.04).
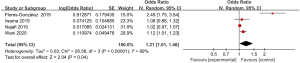
Meta-analysis of the incidence of abdominal pain
A total of 7 articles included in the study reported detailed statistics on the incidence of abdominal pain after digestive endoscopy in children (Figure 4). First, the heterogeneity analysis in the incidence of abdominal pain after digestive endoscopy in children showed an obvious heterogeneity in the incidence of abdominal pain (I2=84%; P<0.00001), so a REM was employed for statistical analysis. The OR value of abdominal pain after digestive endoscopy was 1.18 (95% CI: 1.11 to 1.27), and there was significant heterogeneity in the incidence of abdominal pain after digestive endoscopy in children (Z=4.86; P<0.00001).
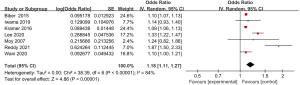
Meta-analysis of the incidence of fever
Three articles included in the study reported detailed statistics on the incidence of fever after digestive endoscopy in children (Figure 5). The heterogeneity in the incidence of fever after digestive endoscopy in children was analyzed, but no heterogeneity was revealed (I2=0%; P=0.65), so a FEM was utilized to analyze the pooled effect values. The OR value of fever after digestive endoscopy in children was 1.09 (95% CI: 1.06 to 1.12), and considerable heterogeneity was indicated in the incidence of fever after digestive endoscopy in children (Z=5.94; P<0.00001).
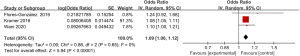
Meta-analysis of incidence of bleeding
Three articles included in the study reported detailed statistics on the incidence of bleeding after digestive endoscopy in children (Figure 6). The analysis of the heterogeneity in the incidence of bleeding after digestive endoscopy in children showed notable heterogeneity (I2=90%; P<0.0001), so a REM was adopted for statistical analysis of pooled effects values. The results showed that the OR value of adverse bleeding reaction after digestive endoscopy in children was 1.24 (95% CI: 0.94 to 1.64), and no great heterogeneity was found in the incidence of bleeding after digestive endoscopy in children (Z=1.53; P=0.13).
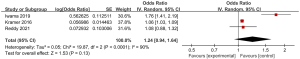
Meta-analysis of incidence of chest pain
A total of 3 articles included in the study reported detailed statistics on the incidence of chest pain after digestive endoscopy in children (Figure 7). Analysis of the heterogeneity in the incidences of chest pain after digestive endoscopy in children revealed no heterogeneity (I2=0%; P=0.73), so a FEM was used for statistical analysis of pooled effect sizes. The OR value of chest pain after gastrointestinal endoscopy in children was 1.06 (95% CI: 1.03 to 1.09), and obvious heterogeneity was found in the incidence of chest pain after digestive endoscopy in children (Z=4.29; P<0.0001).
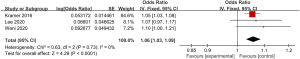
Meta-analysis of the incidence of sore throat
A comparative analysis of the incidence of sore throat among the participants included in the 4 relevant articles was conducted in Figure 8. The incidence of sore throat after digestive endoscopy was first analyzed, and remarkable heterogeneity was found (I2=82%; P=0.0009), so a REM was used for statistical analysis of pooled effect size. The results showed that the OR was 1.11 (95% CI: 1.05 to 1.18) for the occurrence of sore throat after digestive endoscopy in children, and there was a notable difference in the incidence of sore throat after digestive endoscopy in children (Z=3.57; P=0.0004).

Meta-analysis of the incidence of vomiting
Ten articles included in the study reported detailed statistics on the incidence of vomiting after digestive endoscopy in children (Figure 9). The incidence of vomiting after digestive endoscopy in children was analyzed, and there was substantial heterogeneity in the incidence of vomiting (I2=77%; P<0.0001), so a REM was employed for statistical analysis of combined effect values, which showed that the OR value of vomiting after digestive endoscopy in children was 1.13 (95% CI: 1.06 to 1.21), and remarkable heterogeneity in the incidence of vomiting was found after digestive endoscopy in children (Z=3.81; P=0.0001).
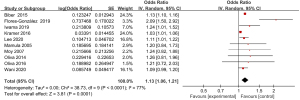
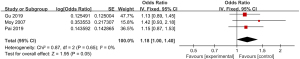
Meta-analysis of delayed capsule expulsion rate
A total of 3 of the included articles reported detailed statistics on delayed capsule expulsion after CE (Figure 10). First, the heterogeneity in the incidence of delayed capsule expulsion after CE was analyzed, and no heterogeneity was indicated between the incidence of delayed capsule expulsion (I2=0%; P=0.65), so a FEM was adopted. The combined effect statistical model of meta-analysis showed that the OR of delayed capsule expulsion after digestive endoscopy in children was 1.18 (95% CI: 1.00 to 1.40), and the incidence of delayed capsule expulsion after digestive endoscopy in children showed no heterogeneity (Z=1.95; P=0.05).
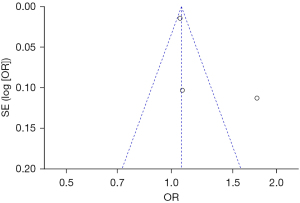
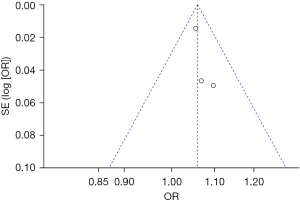
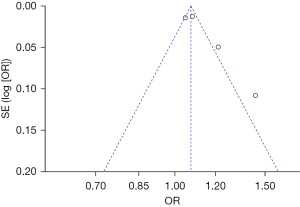
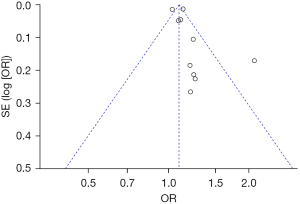
Bias analysis of publications
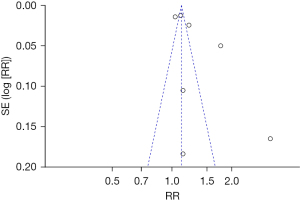
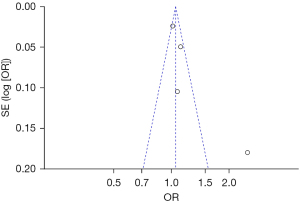
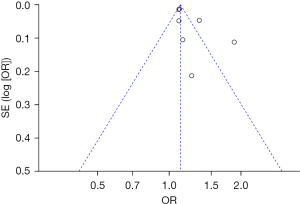
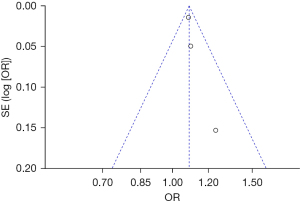
The publication bias analysis of the incidence of AEs after digestive endoscopy in children was performed by drawing an inverted funnel plot, and the results are shown in Figures 11-19. It can be inferred that the funnel plots of the incidence of each AE were relatively symmetrical, and almost all studies fell within the funnel plot. Only the incidence of adverse respiratory events and the incidence of abdominal pain involved individual studies that did not fall into the inverted funnel map. Overall, the almost all incidences of AEs after digestive endoscopy were close to the central axis. All Egger test P values were greater than 0.05, indicating no notable publication bias in the included studies. Based on this, it was shown the incidence of AEs included in this article for analysis had low publication bias and met the requirements.
Discussion
Digestive endoscopy is an imperative approach for digestive system diseases. Affected by physical stimulation during traditional digestive endoscopy, patients are prone to nausea, vomiting, coughing, and other discomforts. In severe cases, arrhythmia and even circulatory respiratory failure can be caused by the high excitation of the vagus nerve (32). A study has pointed out that bowel stretch during colonoscopy can cause clinical symptoms such as abdominal distention, abdominal pain, and nausea (33). In recent years, with the development and wide application of anesthesia gastroenteroscopy technology, the pain of patients during the examination process has been significantly reduced, and the tension and fear of the examinee have been eliminated, so patients and physicians are more comfortable during exams (34). However, gastroenteroscopy anesthesia also produces many adverse reactions and risks due to anesthesia during clinical application. Most of the adverse reactions after gastroenteroscopy related to anesthesia can occur during or after gastroenteroscopy. The AEs include cardiovascular complications such as hypotension, hypertension, arrhythmia, myocardial ischemia or infarction, respiratory depression, airway obstruction, hypoxia, and aspiration (35). A study has pointed out that allergic reactions, hypotension or shock, nausea, vomiting, and paradoxical reactions (aggression, emotional agitation, talkativeness, disorientation, and tachycardia) may also occur after digestive endoscopy (36). Rondonotti et al. [2018] (37) pointed out that after gastrointestinal endoscopy, patients may experience AEs such as cough, hypotension, hypoxemia, abnormal excitement, apnea, and respiratory depression. Both traditional digestive endoscopy and painless digestive endoscopy can cause AEs in patients.
With the continuous development of society and the economy, people’s living and eating habits have changed, and previously adult diseases have gradually begun to affect the younger population (38). Some diseases of the digestive tract cannot be diagnosed by clinical methods alone, and need to be diagnosed by gastrointestinal endoscopy. At present, digestive endoscopy has been widely used in diagnosis and treatment of children with celiac disease, esophageal stricture, eosinophilic esophagitis, inflammatory bowel disease, congenital muscular hypertrophy and lymphoma, congenital small intestinal lymphangiectasia, polyposis disease, congenital hypertrophic pyloric stenosis, and Hirschsprung’s disease (39). The respiratory and circulatory systems are the main sites of AEs in children after GI endoscopy, including fluctuations in heart rate and blood pressure, arrhythmia, myocardial ischemia, respiratory depression, hypoxemia, pulmonary edema, and aspiration (40). At the same time, a study has pointed out that perforation and bleeding, agitation, and hypoglycemia are also associated with AEs after digestive endoscopy in children (41). The results revealed that the incidence of adverse respiratory events after digestive endoscopy was 1.31 (95% CI: 1.17 to 1.47); the incidence of myoclonus was 1.21 (95% CI: 1.01 to 1.46); the incidence of abdominal pain was 1.18 (95% CI: 1.11 to 4.86); the incidence of fever was 1.09 (95% CI: 1.06 to 1.12); the incidence of bleeding was 1.24 (95% CI: 0.94 to 1.64); the incidence of chest pain was 1.06 (95% CI: 1.03 to 1.09); the incidence of sore throat was 1.11 (95% CI: 1.05 to 1.18); the incidence of vomiting was 1.13 (95% CI: 1.06 to 1.21); and the incidence of delayed capsule discharge was 1.18 (95% CI: 1.00 to 1.40). Therefore, the incidence of major AEs included in this work was lower, and the incidence of major AEs was low. Such results indicate that the incidence of AEs after digestive endoscopy in children is low and relatively safe. However, the number of included articles was low and the time span was large, the quality of the article was different, most of the included articles were concerning the application value of digestive endoscopy in the treatment of children’s digestive system diseases, and few articles specifically addressed safety. Therefore, the selection of AEs may have certain deviations. In the future work, massive clinical trials will be included to verify the safety of different digestive endoscopy techniques in treating digestive system diseases in children.
Conclusions
Through meta-analysis, this study explored the incidence of AEs after digestive endoscopy in children. It was found that the incidence of adverse respiratory events, myoclonus, abdominal pain, fever, bleeding, chest pain, sore throat, vomiting, and delayed capsule expulsion after examination were obviously low. This indicated that the use of digestive endoscopy in children’s digestive system diseases was safe and had potential application value. This study lays a theoretical basis for the clinical diagnosis and treatment of digestive system diseases in children.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-179/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-179/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Candia R. Classifications and scores used in digestive endoscopy. Rev Med Chil 2020;148:992-1003. [Crossref] [PubMed]
- Tringali A, Thomson M, Dumonceau JM, et al. Pediatric gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Guideline Executive summary. Endoscopy 2017;49:83-91. [PubMed]
- Lirio RA. Management of Upper Gastrointestinal Bleeding in Children: Variceal and Nonvariceal. Gastrointest Endosc Clin N Am 2016;26:63-73. [Crossref] [PubMed]
- Thomson M, Tringali A, Dumonceau JM, et al. Paediatric Gastrointestinal Endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr 2017;64:133-53. [Crossref] [PubMed]
- Mezoff EA, Williams KC, Erdman SH. Gastrointestinal Endoscopy in the Neonate. Clin Perinatol 2020;47:413-22. [Crossref] [PubMed]
- Tringali A, Balassone V, De Angelis P, et al. Complications in pediatric endoscopy. Best Pract Res Clin Gastroenterol 2016;30:825-39. [Crossref] [PubMed]
- Oliva S, Cohen SA, Di Nardo G, et al. Capsule endoscopy in pediatrics: a 10-years journey. World J Gastroenterol 2014;20:16603-8. [Crossref] [PubMed]
- Oliva S, Cucchiara S, Cohen SA. Recent advances in pediatric gastrointestinal endoscopy: an overview. Expert Rev Gastroenterol Hepatol 2017;11:643-50. [Crossref] [PubMed]
- Tagawa M, Morita A, Imagawa K, et al. Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound in children. Dig Endosc 2021;33:1045-58. [Crossref] [PubMed]
- Johnson KD, Perisetti A, Tharian B, et al. Endoscopic Retrograde Cholangiopancreatography-Related Complications and Their Management Strategies: A "Scoping" Literature Review. Dig Dis Sci 2020;65:361-75. [Crossref] [PubMed]
- Schepers NJ, Hallensleben NDL, Besselink MG, et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): a multicentre randomised controlled trial. Lancet 2020;396:167-76. [Crossref] [PubMed]
- Jackson KL, Goel S, Kho AN, et al. Distance from hospital impacts adverse event detection after outpatient endoscopy. Gastrointest Endosc 2017;85:380-6. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc 2019;89:1075-105.e15. [Crossref] [PubMed]
- Wu Z, Wang S, Yuan S, et al. Clinical efficacy and safety of somatostatin in the treatment of early postoperative inflammatory small bowel obstruction: A protocol for systematic review and meta analysis. Medicine (Baltimore) 2020;99:e20288. [Crossref] [PubMed]
- Benatar JR, Stewart RAH. Cardiometabolic risk factors in vegans; A meta-analysis of observational studies. PLoS One 2018;13:e0209086. [Crossref] [PubMed]
- Archbold RA. Comparison between National Institute for Health and Care Excellence (NICE) and European Society of Cardiology (ESC) guidelines for the diagnosis and management of stable angina: implications for clinical practice. Open Heart 2016;3:e000406. [Crossref] [PubMed]
- Biber JL, Allareddy V, Allareddy V, et al. Prevalence and Predictors of Adverse Events during Procedural Sedation Anesthesia-Outside the Operating Room for Esophagogastroduodenoscopy and Colonoscopy in Children: Age Is an Independent Predictor of Outcomes. Pediatr Crit Care Med 2015;16:e251-9. [Crossref] [PubMed]
- Flores-González JC, Estalella-Mendoza A, Rodríguez-Campoy P, et al. Topical Pharyngeal Lidocaine Reduces Respiratory Adverse Events During Upper Gastrointestinal Endoscopies Under Ketamine Sedation in Children. Paediatr Drugs 2019;21:25-31. [Crossref] [PubMed]
- Gu Z, Wang Y, Lin K, et al. Magnetically Controlled Capsule Endoscopy in Children: A Single-center, Retrospective Cohort Study. J Pediatr Gastroenterol Nutr 2019;69:13-7. [Crossref] [PubMed]
- Iwama I, Shimizu H, Nambu R, et al. Efficacy and safety of a capsule endoscope delivery device in children. Eur J Gastroenterol Hepatol 2019;31:1502-7. [Crossref] [PubMed]
- Kramer RE, Narkewicz MR. Adverse Events Following Gastrointestinal Endoscopy in Children: Classifications, Characterizations, and Implications. J Pediatr Gastroenterol Nutr 2016;62:828-33. [Crossref] [PubMed]
- Lee FC, Queliza K, Chumpitazi BP, et al. Outcomes of Non-anesthesiologist-Administered Propofol in Pediatric Gastroenterology Procedures. Front Pediatr 2020;8:619139. [Crossref] [PubMed]
- Mamula P, Markowitz JE, Neiswender K, et al. Success rate and duration of paediatric outpatient colonoscopy. Dig Liver Dis 2005;37:877-81. [Crossref] [PubMed]
- Moy L, Levine J. Wireless capsule endoscopy in the pediatric age group: experience and complications. J Pediatr Gastroenterol Nutr 2007;44:516-20. [Crossref] [PubMed]
- Najafi N, Veyckemans F, Vanhonacker D, et al. Incidence and risk factors for adverse events during monitored anaesthesia care for gastrointestinal endoscopy in children: A prospective observational study. Eur J Anaesthesiol 2019;36:390-9. [Crossref] [PubMed]
- Oliva S, Cucchiara S, Civitelli F, et al. Colon capsule endoscopy compared with other modalities in the evaluation of pediatric Crohn's disease of the small bowel and colon. Gastrointest Endosc 2016;83:975-83. [Crossref] [PubMed]
- Oliva S, Di Nardo G, Hassan C, et al. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy 2014;46:485-92. [Crossref] [PubMed]
- Pai AK, Jonas MM, Fox VL. Esophageal Capsule Endoscopy in Children and Young Adults With Portal Hypertension. J Pediatr Gastroenterol Nutr 2019;69:641-7. [Crossref] [PubMed]
- Reddy PM, Kulkarni S, Nabi Z, et al. Single balloon enteroscopy in children for evaluation of small bowel diseases in children: A large, tertiary center study. J Pediatr Surg 2021;56:2005-9. [Crossref] [PubMed]
- Sanders K, Osterbauer B, Forman N, et al. Perioperative respiratory adverse events in children undergoing triple endoscopy. Paediatr Anaesth 2021;31:1290-7. [Crossref] [PubMed]
- Wani MA, Zargar SA, Yatoo GN, et al. Endoscopic Yield, Appropriateness, and Complications of Pediatric Upper Gastrointestinal Endoscopy in an Adult Suite: A Retrospective Study of 822 Children. Clin Endosc 2020;53:436-42. [Crossref] [PubMed]
- Antao B, Bishop J, Shawis R, et al. Clinical application and diagnostic yield of wireless capsule endoscopy in children. J Laparoendosc Adv Surg Tech A 2007;17:364-70. [Crossref] [PubMed]
- Broide E, Shalem T, Richter V, et al. The Safety and Feasibility of a New Through-the-scope Balloon-assisted Enteroscopy in Children. J Pediatr Gastroenterol Nutr 2020;71:e6-e11. [Crossref] [PubMed]
- Kaur S, Rosen JM, Kriegermeier AA, et al. Utility of Gastric and Duodenal Biopsies During Follow-up Endoscopy in Children With Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2017;65:399-403. [Crossref] [PubMed]
- Mahajan R, Simon EG, Chacko A, et al. Endoscopic ultrasonography in pediatric patients--Experience from a tertiary care center in India. Indian J Gastroenterol 2016;35:14-9. [Crossref] [PubMed]
- van Beek EJ, Leroy PL. Safe and effective procedural sedation for gastrointestinal endoscopy in children. J Pediatr Gastroenterol Nutr 2012;54:171-85. [Crossref] [PubMed]
- Rondonotti E, Spada C, Adler S, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2018;50:423-46. [Crossref] [PubMed]
- Spagnuolo R, Corea A, Blumetti M, et al. Effects of listening to music in digestive endoscopy: A prospective intervention study led by nursing. J Adv Nurs 2020;76:2993-3002. [Crossref] [PubMed]
- Kudo T, Abukawa D, Nakayama Y, et al. Nationwide survey of pediatric gastrointestinal endoscopy in Japan. J Gastroenterol Hepatol 2021;36:1545-9. [Crossref] [PubMed]
- Uchida D, Tsutsumi K, Kato H, et al. Potential Factors Affecting Results of Short-Type Double-Balloon Endoscope-Assisted Endoscopic Retrograde Cholangiopancreatography. Dig Dis Sci 2020;65:1460-70. [Crossref] [PubMed]
- Pécsi D, Farkas N, Hegyi P, et al. Transpancreatic sphincterotomy has a higher cannulation success rate than needle-knife precut papillotomy - a meta-analysis. Endoscopy 2017;49:874-87. [Crossref] [PubMed]



