
Efficacy and safety of Qinxiang Qingjie oral solution for the treatment of influenza in children: a randomized, double-blind, multicenter clinical trial
Introduction
Influenza is an acute contagious respiratory disease caused by influenza viruses and can result in seasonal epidemics and pandemics; thus, it is a serious global health problem (1,2). The influenza A subtypes H1N1 and H3N2 as well as the influenza B subtypes Victoria and Yamagata are the most commonly detected influenza viruses, causing uncomplicated symptoms such as fever (39−40 ℃), chills, headache, myalgia, fatigue, and loss of appetite; respiratory-tract symptoms such as cough, sore throat, runny nose, and nasal obstruction may also result (1). Each year, approximately 20–30% of children, the high-risk population for influenza (1,2), experience seasonal influenza epidemics, with an annual infection rate of up to 50% worldwide (3-5). Furthermore, about 30% of children develop influenza complications, with children under the age of five years old and infants under the age of two years old at an increased risk of developing severe cases with complications (2,6). Evidence has demonstrated that influenza accounts for 10–15% of pediatric hospitalizations each year, resulting in increased mortality as well as massive social and economic burdens (7). A 3-year epidemiological study conducted in Hong Kong has demonstrated that influenza caused a total of 662–1,046 days of school absence and 214–336 days of parental work loss per 10,000 children younger than 18 years old per year; moreover, on average, each school-aged child was absent from school for five days due to influenza and the cost of hospitalization due to influenza was $1,300 per person (8).
Four classes of anti-influenza drugs, including neuraminidase inhibitors, M2 ion channel blockers, hemagglutinin inhibitors, and RNA polymerase inhibitors are available (2,9). However, circulating influenza virus strains have been found to be resistant to M2 ion channel blockers such as amantadine and rimantadine. Meanwhile, there is scant evidence to support the clinical use of the hemagglutinin inhibitor umifenovir in children (1). In China, RNA polymerase inhibitors such as baloxavir and favipiravir are not approved. Although the oral drug oseltamivir is considered a first-line drug and an optimal treatment for pediatric influenza (10), the oseltamivir-resistant seasonal influenza A virus H1N1 has rapidly spread throughout the world (11,12). In addition, oseltamivir causes gastrointestinal, mental, and neurological adverse events, which can result in patient deterioration and death (13-15).
Therefore, the exploration of novel influenza treatments has also gained attention in traditional Chinese medicine (TCM). The TCM treatment of influenza is multi-pronged, and includes combating influenza viruses, enhancing the immune response, and achieving satisfactory clinical outcomes with less drug resistance and fewer adverse drug reactions (16). Furthermore, TCM has antibacterial, antipyretic, and analgesic properties (16), implying that the development of TCMs for treating influenza in children is clinically significant.
Qinxiang Qingjie (QXQJ) oral solution is a traditional Chinese herb preparation, which is composed of Huang qin (Radix scutellariae), Guang huo xiang (Patchouli), Chan tui (Periostracum cicadae), Shi gao (Gypsum fibrosum), Ge gen (Kudzu Root), Da huang (Radix et Rhizoma Rhei), Chi shao (Radix Paeoniae Rubra), Ban lan gen (Radix isatidis), Jie geng (Radix platycodi), Xuan shen (Radix scrophulariae), Shan dou gen (Sophora subprostrata), and Gan cao (Radix glycyrrhizae). It is used for dispersing wind-heat, clearing the internal heat, detoxification, and relieving a sore throat. A previous study has reported antipyretic, analgesic, antitussive, expectorant, anti-inflammatory, and antibacterial effects of QXQJ (17). In vitro studies found that patchouli alcohol, polyphenolic compounds from Patchouli and baicalin, Flavonoids-enriched extract from Scutellaria baicalensis root could exert some inhibitory effects on the virus. The acute and long-term toxicity reactions suggest that QXQJ has low toxicity and high safety (18). Chinese medicine is multi-component and multi-targeted, acting integrally on the body rather than having a single effect. Compared to western chemical antiviral western chemical antiviral agents, herbal medicine has potential advantages in restoring the body’s functional balance and improving overall symptoms with a higher safety profile (19). However, due to the complex composition, the chemical composition and mechanism of action of QXQJ have not been adequately studied. It is widely used to treat upper respiratory tract infections in children with TCM syndromes of both the exterior and interior heat types (20-22). However, there is a lack of scientific evidence supporting the use of QXQJ to treat pediatric influenza. To address this gap, we conducted a randomized, double-blind, double-dummy, positive-controlled, multicenter, noninferiority clinical study comparing the efficacy and safety of the QXQJ oral solution to those of oseltamivir in children with influenza.
We present the following article in accordance with the CONSORT reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-201/rc).
Methods
General information
From March 2019 to May 2020, this randomized, double-blind, double-dummy, positive-controlled, multicenter clinical trial was conducted in 14 hospitals throughout China in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice (23). The study protocol was reviewed and approved by the Ethics Committee of the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (No. TYLL2018 [Y] 019). All other participating hospitals (Maternity and Child Health Care of Zaozhuang, The First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, Shanghai Children’s Medical Center, Ezhou Central Hospital, The First Affiliated Hospital of Henan University of Chinese Medicine, Shanghai Municipal Hospital of Traditional Chinese Medicine, Handan Hospital of Traditional Chinese Medicine, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Maternal and Child Health Care Hospital of Yuncheng, Taiyuan Maternity and Child Health Care Hospital, Dongfang Hospital Beijing University of Chinese Medicine, Luohe Hospital of Traditional Chinese Medicine, Changzhi People’s Hospital) were informed and agreed with this study. All parents/guardians of the patients provided written informed consent before enrollment. Participants over the age of 8 also gave written consent after understanding the risks and benefits of the study.
Participants
The inclusion criteria for eligibility were as follows: (I) aged 1–13 years; (II) met both the diagnostic criteria for influenza (24) and TCM syndromes of the exterior and interior heat types (25); and (III) developed a fever with an axillary temperature of ≥38 ℃ in 48 hours. The exclusion criteria were as follows: (I) pharyngoconjunctival fever, herpangina, or suppurative tonsillitis; (II) complications of sinusitis, otitis media, bronchitis, or pneumonia; (III) severe or critical influenza; (IV) received antiviral drugs within 48 hours prior to enrollment; (V) received an influenza vaccination within 12 months before enrollment; (VI) currently receiving systemic corticosteroids or immunosuppressive therapy; (VII) history of epilepsy, febrile convulsions, or recurrent respiratory infections; (VIII) severe malnutrition or rickets, or severe primary heart, brain, liver, kidney, or hematopoietic disease; (IX) allergic to the study drugs or prone to develop allergic reactions (allergic to ≥2 foods or drugs); and (X) lost to follow-up.
Sample size
This trial aimed to add a new functional specialty of QXQJ. According to the Drug Registration Regulation issued by National Medical Products Administration in 2007, the sample size of each group must be at least 100 cases. We finally designed to enroll 240 patients, with 120 for each group, considering a dropout rate of 20%.
Randomization, allocation concealment, and blinding
A random sequence with a 1:1 allocation ratio to the QXQJ or oseltamivir group was generated using SAS software (SAS Inc., Cary, NC, USA). The clinical setting was considered as the stratification factor. Personnel unrelated to this clinical trial performed the randomization procedures, assigned the participants, and prepared the random codes and emergency letters. After the medications were coded, they were sealed and blindly stored until completion of the study. All patients, investigators, and statisticians were unaware of the group assignment. The first unblinding was performed after verifying and locking the database, and the second unblinding occurred following the completion of the statistical analysis report. The investigators properly retained the emergency letters distributed to the centers along with the medications. Only in an emergency, when the participant’s medication situation required clarification, could the blinding be uncovered urgently. Following the opening of the emergency letter, the signer, the date of the opening, and the reason for the opening needed to be indicated.
After producing the placebo, the sponsor invited some people to taste whether it was difficult to distinguish between the placebo and the herbal and control drugs. We used the same packaging and coded the medications according to a random number table. Researchers dispensed the medicines in order during the trial. The blinding code was managed by third-party personnel not involved in test trials. After the study was completed, we confirmed the integrity of the blinding code and checked if the emergency letters had been opened illegally.
Interventions
The corresponding simulants were prepared consistently with QXQJ and oseltamivir in terms of the appearance, odor, and taste. Sucrose and purified water were used to make the QXQJ simulant. Sucrose, sodium carboxymethyl cellulose, and purified water were the components of the oseltamivir simulant. The administered doses of QXQJ and the corresponding simulant were as follows: 10 mL for children aged 1–2 years old, 15 mL for those aged 3–6 years old, and 20 mL for those aged 7–13 years old; four times daily for children with a fever or three times daily when the children were fever-free, for five days. Oseltamivir phosphate granule and the corresponding simulant were administered based on body weight: 30 mg (≤15 kg), 45 mg (15–23 kg), 60 mg (23–40 kg), and 75 mg (>40 kg or age ≥13 years old); orally twice daily for 5 days.
Guangzhou ApicHope Pharmaceutical Co., Ltd., Guangdong, China, provided the study interventions, which were distributed through each setting’s central pharmacy.
Protocol
The study was divided into two phases: a 5-day treatment period and a 2-day medical observation period. The participants were assigned randomly to receive either QXQJ oral solution or oseltamivir, but no other specific influenza medications or therapies were allowed, with the exception of physical cooling. For patients with an axillary temperature of >38.5 ℃, paracetamol was prescribed at a maximum dose of 10 mg/kg every 4–6 hours, as needed, and no more than four times daily with the investigator’s consent. If the fever persisted, additional antipyretic and analgesic medications could be prescribed. In the case report forms and the medical records, the generic names, administration times, dosages, and reasons for prescribing the concomitant medications were recorded. The follow-up visits occurred on days 5 and 7 following treatment initiation. During the medical observation period, the patient diary and the Canadian Acute Respiratory Illness Flu Scale (CARIFS) (26,27) were recorded by the caregivers every 24 hours. In addition, the axillary temperature was measured every 6 hours. We used the verified and adjusted Chinese version of CARIFs in this study (27). The Chinese version of CARIFs differs slightly from the English version in that it includes 16 items covering three domains: symptoms (headache, sore throat, muscle aches or pain, fever, cough, nasal congestion, runny nose, vomiting), function (poor appetite, not sleeping well, irritable/cranky/fussy, feels unwell, low energy, tired, unable to get out of bed), and the parental impact (crying more than usual, needing extra care, clinging). The 4-point ordinal scale was as follows: 0= none, 1= mild, 2= moderate, 3= severe. Considering the limited comprehension and expression abilities of infants younger than 2 years old, the four items of feels unwell, headache, sore throat, and muscle aches or pain can be evaluated as “do not know” or “not applicable”. The final CARIFS score was calculated by adding the scores of all applicable items.
Efficacy evaluation
The primary study endpoint was the clinical recovery time, defined as complete defervescence for at least 24 hours and a CARIFS symptom dimension score of 0 or 1. The secondary study endpoints were as follows: (I) time to defervescence, defined as the time period during which a participant’s axillary temperature dropped to 37.2 ℃ for the first time in the following 24 hours; (II) incidences of complications (such as sinusitis, otitis media, bronchitis, pneumonia, or hospitalization due to influenza) and severe or critical influenza (24); (III) negative conversion rate, which referred to the rate of conversion to testing influenza negative within five days; and (IV) improvement in the interior and exterior heat types of TCM syndromes. According to the TCM criteria detailed (see Tables S1,S2), clinical efficacy was classified as clinical recovery, remarkable improvement, effective, or ineffective, using the gradational scoring method (25).
Safety evaluation
Safety profiles, including adverse clinical events and adverse drug reactions, were evaluated by physical examination (body temperature, resting heart rate, breathing rate, and blood pressure), electrocardiogram, and laboratory tests (white blood cell count, red blood cell count, neutrophil %, lymphocyte %, hemoglobin, platelet, C-reactive protein, alanine aminotransferase, aspartate aminotransferase, total bilirubin, gamma-glutamyl transferase, blood urea nitrogen, creatinine, urine white blood cell count, urine red blood cell count, and protein in urine).
Statistical analysis
The software SAS 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. A P value <0.05 was considered statistically significant. Normally distributed quantitative data were presented as the mean and standard deviation, and intergroup comparisons were performed by the t-test. The analysis of covariance, including the test center, influenza virus strain type, and patient baseline data, was also conducted. Meanwhile, non-normally distributed data were presented as the median and analyzed using the Kruskal-Wallis rank-sum test. Qualitative data were expressed as numbers and percentages and analyzed by the chi-squared test or Fisher’s exact test.
Efficacy was assessed by the full analysis set (FAS) and the per protocol set (PPS), whereas safety evaluation was analyzed using the safety set (SS). The last observation carried forward approach was utilized for dealing with missing values in the primary outcome. Considering other factors such as the clinical setting, the Cochran-Mantel-Haenszel chi-squared test was added to the analyses. The Wilcoxon rank sum test was considered when comparing the ranked data in multiple settings. Kaplan-Meier curves of survival data were used to describe the censoring rate and the survival time for both groups, and then the log-rank test was conducted for comparison. The accelerated failure time model was adopted because some important non-treatment factors (such as the clinical setting, baseline characteristics, and the course of disease) had an impact on the primary outcome. According to the minimum information principle, the model with the best fitting degree was chosen to calculate the ratio of the median recovery time between the two groups and the 95% confidence interval (CI). According to a previous study, the median recovery time for children with influenza was four days (101 h) for oseltamivir and less than one day for QXQJ oral solution, indicating that oseltamivir was not inferior to QXQJ (28). Thus, the lower limit of the 95% CI of the median recovery time ratio was set as >0.8.
Results
Baseline characteristics
A total of 231 enrolled patients were randomly assigned to receive either QXQJ oral solution (n=117) or oseltamivir (n=114) from March 2019 to May 2020. As shown in the Consolidated Standards of Reporting Trials (CONSORT) flowchart (Figure 1), 224 patients were included in the FAS analysis (n=112/group), 212 in the PPS analysis (n=106/group), and 225 in the SS analysis (n=113 for QXQJ, n=112 for oseltamivir). As shown by the consistent results of the FAS and PPS analyses (Table 1), there were no significant differences in the baseline characteristics between the two groups.
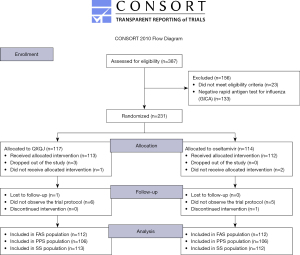
Table 1
| Baseline | FAS analysis | PPS analysis | |||||
|---|---|---|---|---|---|---|---|
| QXQJ (n=112) | Oseltamivir (n=112) | P value | QXQJ (n=106) | Oseltamivir (n=106) | P value | ||
| Age, mean ± SD, y | 7.019±3.025 | 6.629±2.670 | 0.3073a | 7.043±3.085 | 6.567±2.654 | 0.2298a | |
| Height, mean ± SD, cm | 121.201±20.147 | 118.605±18.399 | 0.3151a | 121.288±20.214 | 118.413±18.314 | 0.2792a | |
| Weight, mean ± SD, kg | 25.432±10.347 | 23.836±8.657 | 0.2118a | 25.758±10.497 | 23.657±8.559 | 0.1116a | |
| Gender, n (%) | |||||||
| Male | 60 (53.57) | 54 (48.21) | 0.4226b | 58 (54.72) | 50 (47.17) | 0.2717b | |
| Female | 52 (46.43) | 58 (51.79) | 48 (45.28) | 56 (52.83) | |||
| Ethnicity, n (%) | |||||||
| Han | 111 (99.11) | 111 (99.11) | 1.0000c | 106 (100.0) | 105 (99.06) | 1.0000c | |
| Others | 1 (0.89) | 1 (0.89) | 0 (0.00) | 1 (0.94) | |||
| Type A flu, n (%) | |||||||
| Positive | 103 (91.96) | 100 (89.29) | 0.4917b | 99 (93.40) | 95 (89.62) | 0.3243b | |
| Negative | 9 (8.04) | 12 (10.71) | 7 (6.60) | 11 (10.38) | |||
| Type B flu, n (%) | |||||||
| Positive | 11 (9.82) | 12 (10.71) | 0.8258b | 9 (8.49) | 11 (10.38) | 0.6384b | |
| Negative | 101 (90.18) | 100 (89.29) | 97 (91.51) | 95 (89.62) | |||
| Flu classification, n (%) | |||||||
| A + B | 2 (1.79) | 0 (0.00) | 0.4066c | 2 (1.89) | 0 (0.00) | 0.2706c | |
| A | 101 (90.18) | 100 (89.29) | 97 (91.51) | 95 (89.62) | |||
| B | 9 (8.04) | 12 (10.71) | 7 (6.60) | 11 (10.38) | |||
| Course of disease, mean ± SD, h | 19.232±11.641 | 20.179±13.439 | 0.5738a | 18.868±11.477 | 20.623±13.629 | 0.3118a | |
| Pre-diagnostic Tmax, mean ± SD, ℃ | 38.887±0.652 | 38.819±0.586 | 0.4134a | 38.869±0.645 | 38.822±0.587 | 0.5783a | |
| Family history, n (%) | |||||||
| Yes | 0 (0.00) | 0 (0.00) | – | 0 (0.00) | 0 (0.00) | – | |
| No | 112 (100.0) | 112 (100.0) | 106 (100.0) | 106 (100.0) | |||
| History of allergy, n (%) | |||||||
| Yes | 3 (2.68) | 0 (0.00) | 0.2466c | 2 (1.89) | 0 (0.00) | 0.4976c | |
| No | 109 (97.32) | 112 (100.0) | 104 (98.11) | 106 (100.0) | |||
| Medical history, n (%) | |||||||
| Yes | 1 (0.89) | 5 (4.46) | 0.2124c | 0 (0.00%) | 5 (4.72) | 0.0596c | |
| No | 111 (99.11) | 107 (95.54) | 106 (100.0) | 101 (95.28) | |||
| Pre-diagnostic treatment, n (%) | |||||||
| Yes | 40 (35.71) | 47 (41.96) | 0.3372b | 38 (35.85) | 43 (40.57) | 0.4797b | |
| No | 72 (64.29) | 65 (58.04) | 68 (64.15) | 63 (59.43) | |||
| CARIFS score, mean ± SD | 21.482±10.285 | 20.696±7.786 | 0.5199a | 21.594±10.491 | 20.849±7.779 | 0.5575a | |
| TCM syndrome score, mean ± SD | 7.286±2.729 | 7.482±2.695 | 0.5884a | 7.358±2.751 | 7.538±2.709 | 0.6331a | |
a, t-test; b, χ2 text; c, Fisher’s exact test. QXQJ, Qinxiang Qingjie; FAS, full analysis set; PPS, per protocol set; SD, standard deviation; CARIFS, the Canadian Acute Respiratory Illness and Flu Scale; TCM, traditional Chinese medicine; Tmax, maximum temperature.
Primary study endpoints
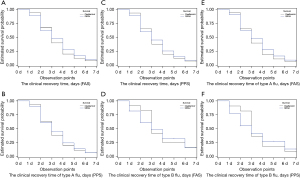
The FAS and PPS results showed that the median clinical recovery time was 3 days in both the QXQJ and oseltamivir groups, with no significant difference (FAS, P=0.5328; PPS, P=0.6995). Subgroup analyses indicated that the median clinical recovery time for patients with type A (FAS, P=0.4417; PPS, P=0.6818) or type B influenza (FAS, P=0.8291; PPS, P=0.8674) was 3 days, with no significant intergroup difference. According to the accelerated failure time-lognormal distribution model, the median time ratio was 0.993 (95% CI: 0.866 to 1.139) for the FAS and 0.983 (95% CI: 0.856 to 1.129) for the PPS, when the clinical setting and disease course were considered. The efficacy of oseltamivir was not inferior to that of the QXQJ oral liquid with a cut-off value of 0.80 (Table 2 and Figure 2).
Table 2
| Efficacy outcomes | FAS analysis | PPS analysis | |||||
|---|---|---|---|---|---|---|---|
| QXQJ (n=112) |
Oseltamivir (n=112) | P value | QXQJ (n=106) |
Oseltamivir (n=106) | P value | ||
| Clinical recovery time (days), Med [Q1–Q3], | 3 [2–5] | 3 [2–4] | 0.5328a | 3 [2–5] | 3 [2–4] | 0.6995a | |
| Time to resolution of fever (hours), Med [Q1–Q3], | 36 [24–54] | 36 [24–54] | 0.2552a | 36 [24–54] | 36 [24–54] | 0.4826a | |
| Incidence rate of complications, n (%) | 5 (4.46%) | 1 (0.89%) | 0.2124b | 4 (3.77%) | 0 (0.00%) | 0.1215b | |
| Incidence rate of severe or critical influenza, n (%) | 0 (0.00%) | 0 (0.00%) | – | 0 (0.00%) | 0 (0.00%) | – | |
| Improvement in TCM syndromes, n (%) | 91 (81.25%) | 96 (85.71%) | 0.3683c | 90 (84.91%) | 94 (88.68%) | 0.4171c | |
| Difference of TCM syndrome scores before and after treatment, mean ± SD |
5.330±3.687 | 5.563±3.187 | 0.6147d | 5.528±3.623 | 5.764±3.051 | 0.6087d | |
a, log-rank; b, Fisher’s exact test; c, Wilcoxon rank sum test; d, t-test. QXQJ, Qinxiang Qingjie; FAS, full analysis set; PPS, per protocol set; TCM, traditional Chinese medicine; SD, standard deviation.
Secondary study endpoints
According to the FAS and PPS analyses, the median time to defervescence was 36 hours in both the QXQJ and oseltamivir groups, with no significant difference (FAS, P=0.2552; PPS, P=0.4826) (Table 2 and Figure 3). The FAS and PPS analyses also showed that the incidence rate of complications was 4.46% and 3.77%, respectively, in the QXQJ group and 0.89% and 0.00%, respectively, in the oseltamivir group, with no remarkable difference (FAS, P=0.2124; PPS, P=0.1215). There was no severe or critical influenza in either group (Table 2). In addition, the FAS and PPS analyses of the improvement in TCM syndromes revealed that the proportion of patients who reported clinical recovery and remarkable improvement was 81.25% and 84.91%, respectively, for QXQJ and 85.71% and 88.68%, respectively, for oseltamivir, without significant differences (FAS, P=0.3683; PPS, P=0.4171) (Table 2). Moreover, the FAS and PPS analyses of the TCM syndrome scores revealed that the difference of the TCM syndrome scores before and after treatment was 5.330±3.687 and 5.528±3.623, respectively, in the QXQJ group and 5.563±3.187 and 5.764±3.051, respectively, in the oseltamivir group, with no significant differences (FAS, P=0.6147; PPS, P=0.6087) (Table 2). As shown in Table 3, after treatment, there are no significant differences in the changes in CARIFs scores at each visit point relative to their baseline values between groups. Furthermore, there were no significant differences in the proportion of patients testing negative for influenza A or influenza B in both groups, with the FAS and PPS analyses showing comparable results (Table 4).
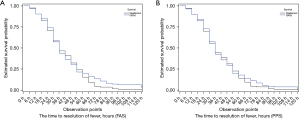
Table 3
| Baseline-to-posttreatment change in CARIFs score | FAS analysis | PPS analysis | |||||
|---|---|---|---|---|---|---|---|
| QXQJ | Oseltamivir | P valuea | QXQJ | Oseltamivir | P valuea | ||
| Day 1 | 7.147±10.014 | 5.198±6.855 | 0.0931 | 7.295±10.156 | 5.472±6.809 | 0.1268 | |
| Day 2 | 11.926±10.127 | 10.655±8.750 | 0.3221 | 12.125±10.185 | 10.952±8.768 | 0.3733 | |
| Day 3 | 15.551±10.745 | 14.946±8.122 | 0.6385 | 15.596±10.853 | 15.217±7.964 | 0.7729 | |
| Day 4 | 17.944±10.460 | 17.648±8.059 | 0.8165 | 18.048±10.570 | 17.798±7.979 | 0.8475 | |
| Day 5 | 19.467±10.379 | 18.745±7.961 | 0.5654 | 19.519±10.500 | 18.906±7.889 | 0.6322 | |
| Day 6 | 20.159±10.331 | 19.236±7.931 | 0.4607 | 20.240±10.441 | 19.377±7.883 | 0.4993 | |
| Day 7 | 20.626±10.155 | 19.627±7.808 | 0.4168 | 20.712±10.258 | 19.726±7.785 | 0.4334 | |
a, t-test. QXQJ, Qinxiang Qingjie; CARIFS, the Canadian Acute Respiratory Illness and Flu Scale; FAS, full analysis set; PPS, per protocol set.
Table 4
| Patients | Database | Results | QXQJ group | Oseltamivir group | P value |
|---|---|---|---|---|---|
| Type A flu (positive), n (%) | FAS | 1 | 57 (55.34) | 55 (55.00) | 1.000 |
| 2 | 2 (1.94) | 1 (1.00) | |||
| 3 | 44 (42.72) | 44 (44.00) | |||
| Total | 103 | 100 | |||
| Type A flu (positive), n (%) | PPS | 1 | 56 (56.57) | 52 (54.74) | 0.8696 |
| 2 | 2 (2.02) | 1 (1.05) | |||
| 3 | 41 (41.41) | 42 (44.21) | |||
| Total | 99 | 95 | |||
| Type B flu (positive), n (%) | FAS | 1 | 3 (27.27) | 6 (50.00) | 0.6802 |
| 2 | 1 (9.09) | 1 (8.33) | |||
| 3 | 7 (63.64) | 5 (41.67) | |||
| Total | 11 | 12 | |||
| Type B flu (positive), n (%) | PPS | 1 | 3 (33.33) | 6 (54.55) | 0.4959 |
| 2 | 1 (11.11) | 0 (0.00) | |||
| 3 | 5 (55.56) | 5 (45.45) | |||
| Total | 9 | 11 | |||
| Influenza (positive), n (%) | FAS | 1 | 58 (51.79) | 61 (54.46) | 0.8655 |
| 2 | 3 (2.68) | 2 (1.79) | |||
| 3 | 51 (45.54) | 49 (43.75) | |||
| Total | 112 | 112 | |||
| Influenza (positive), n (%) | PPS | 1 | 57 (53.77) | 58 (54.72) | 0.7739 |
| 2 | 3 (2.83) | 1 (0.94) | |||
| 3 | 46 (43.40) | 47 (44.34) | |||
| Total | 106 | 106 |
1: positive (baseline) – negative (outcome); 2: positive (baseline) – positive (outcome); 3: positive (baseline) – data missing (outcome). QXQJ, Qinxiang Qingjie; FAS, full analysis set; PPS, per protocol set.
Safety profiles
A total of 28 adverse events, including vomiting, abdominal discomfort, abnormal blood tests, abnormal liver function tests, and complications, were reported, with 14 (12.39%) in the QXQJ group and 14 (12.50%) in the oseltamivir group. One patient (0.88%) who received the QXQJ oral solution was hospitalized due to a serious adverse event of pneumonia, leading to withdrawal from the study. Four adverse drug reactions were observed, including 1 (0.88%) in the QXQJ group and 3 (2.68%) in the oseltamivir group. The incidences of adverse events, serious adverse events, and adverse drug reactions did not differ significantly between the two groups (Table 5). In addition, there were no significant differences in vital sign changes or laboratory test results, with the exception of the platelet count normal/abnormal (baseline)—abnormal (outcome) rate, which was 25% in the oseltamivir group and 10.48% in the QXQJ group (P=0.006).
Table 5
| Safety outcomes | SS analysis | ||
|---|---|---|---|
| QXQJ (n=113) | Oseltamivir (n=112) | P value | |
| AE, n (%) | 14 (12.39) | 14 (12.50) | 0.9799a |
| SAE, n (%) | 1 (0.88) | 0 (0.00) | 1.0000b |
| ADR, n (%) | 1 (0.88) | 3 (2.68) | 0.3694b |
a, χ2 text; b, Fisher’s exact test. AE, adverse event; SAE, serious adverse event; ADR, adverse reaction; SS, safety set; QXQJ, Qinxiang Qingjie.
Discussion
In this clinical trial, we demonstrated that QXQJ was comparable to oseltamivir in terms of efficacy and safety for treating pediatric influenza. This study employed both TCM syndrome scores and laboratory test results to investigate the efficacy of a TCM in a quantitative and comprehensive manner. Our results showed that the clinical recovery time for both the QXQJ and oseltamivir groups was three days, with no significant differences in the incidence of complications, cases of severe or critical influenza, or negative conversion. Moreover, QXQJ oral solution was as safe and acceptable as oseltamivir in terms of adverse events, serious adverse events, and adverse reactions. Both groups experienced common clinical complications associated with influenza, including otitis media, bronchitis, and pneumonia. Due to their immature immune development, children are more susceptible to complications than adults, which suggests that they should be treated for influenza as soon as possible to minimize the risk of complications.
Favorable effects of QXQJ have been extensively reported. A previous pharmacodynamic study demonstrated that the QXQJ oral solution inhibits influenza virus proliferation in chicken embryos at a minimum inhibitory concentration of 0.062 g/mL, indicating its inhibitory effect on viral replication. In addition, QXQJ may reduce the release of inflammatory mediators, thereby lowering cytokine levels and regulating immunity (17). Besides, patchouli alcohol, a monomer derived from patchouli, has been shown to have activities against influenza virus in vitro (29). Similarly, Wu et al. reported that patchouli downregulates the expression of inflammatory cytokines such as interferon-γ (IFN-γ) and interleukin-4 (IL4) (30). According to Liu et al., polyphenolic compounds isolated from patchouli may have potential as novel anti-influenza agents due to their neuraminidase inhibitory activity (31). Radix scutellariae, another component of the QXQJ oral solution, has been confirmed to protect influenza A virus-infected mice by inhibiting neuraminidase activities and remarkably decreasing lung virus titers (32). Another study revealed that Radix scutellariae extracts, namely, baicalin, baicalein, wogonin, chrysin, and oroxylin A, have lower half-maximal inhibitory concentration values than oseltamivir phosphate and that free flavonoids exhibited greater anti-H1N1 effects than O-glycosides and C-glycosides (33). Other ingredients of the QXQJ oral solution have been reported to help patients recover from the flu by inhibiting neuraminidase, attenuating the expression of IL6, reducing reactive oxygen species and nitric oxide production, imposing antipyretic effects, and promoting blood circulation (34-37).
Previous randomized controlled trials have explored the roles of several TCMs, for example, Antiwei granules, Maxingshigan, and Yinqiaosan ban lan gen granules, in the treatment of influenza when compared with placebo and oseltamivir (38-40). These results demonstrate the benefits of TCMs in fever resolution, symptom relief, and flu recovery. As opposed to antiviral drugs like oseltamivir, the clinical value of TCM lies in a comprehensive treatment of influenza from multiple targets and multiple levels. The main objective is to shorten the course of the disease, which is why clinical recovery time is the prime indicator of this medicine, while virological indicator is an important secondary indication. This is the first study to examine the efficacy and safety of QXQJ in children with influenza by introducing a double-dummy technique to enhance clinical operability and minimize bias. In addition, we considered the clinical recovery time together with TCM syndrome scores, creating an objective and comprehensive evaluation system for efficacy. However, several limitations should be acknowledged. For example, there was no placebo-controlled group to evaluate the absolute efficacy of this trial. There are several reasons for not using a placebo control in this study. At first, oseltamivir phosphate remains the first-line treatment option and a recognized positive control drug according to pediatric influenza guidelines in both China and USA. Second, Chinese parents have a low acceptance for placebo, making it difficult to recruit study participants. Furthermore, children are more susceptible to flu complications and severe illness. According to basic medical ethics principles, the use of placebos does not aid in the clinical recovery of children with influenza. Moreover, some participants failed to completely recover by the endpoint of this study due to the short observation period. Although throat swabs were collected from all patients at baseline, only 124 (55.36%) patients had their throat swabbed at the study endpoint. Considering that most children resist the throat swab examination, this study did not force children to undergo sample collection following treatment, resulting in missing data. However, the virus negative conversion rate is a secondary indicator that has no bearing on our primary conclusions.
Conclusions
This study found that the QXQJ oral solution and oseltamivir are equally effective and safe for the treatment of influenza in children.
Acknowledgments
We thank Medjaden Inc. for scientific editing of this manuscript.
Funding: This study was funded by Guangzhou ApicHope Pharmaceutical Co., Ltd. Support for this study was provided by Chinese-Western Medicine Research and Development Working Committee, China Association of Traditional Chinese Medicine.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-201/rc
Trial Protocol: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-201/tp
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-201/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-201/coif). All authors report that this study was funded by Guangzhou ApicHope Pharmaceutical Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice. The study protocol was reviewed and approved by the Ethics Committee of the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (No. TYLL2018 [Y] 019). All the other participating hospitals (Maternity and Child Health Care of Zaozhuang, The First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, Shanghai Children’s Medical Center, Ezhou Central Hospital, The First Affiliated Hospital of Henan University of Chinese Medicine, Shanghai Municipal Hospital of Traditional Chinese Medicine, Handan Hospital of Traditional Chinese Medicine, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Maternal and Child Health Care Hospital of Yuncheng, Taiyuan Maternity and Child Health Care Hospital, Dongfang Hospital Beijing University of Chinese Medicine, Luohe Hospital of Traditional Chinese Medicine, Changzhi People’s Hospital) were informed and agreed with this study. All parents/guardians of the patients provided written informed consent before enrollment. Participants over the age of 8 also gave written consent after understanding the risks and benefits of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Clinical Research Center for Respiratory Disease, Chinese Pediatric Society, Chinese Medical Association. Expert consensus on diagnosis and treatment of influenza in children (2020 Edition). Chin J Appl Clin Pediatr 2020;35:1281-8.
- National Health Commission of the People’s Republic of China [Internet]. Guidelines for Diagnosis and Treatment of Influenza 2020. [cited 2021 July 1]. Available online: http://www.gov.cn/zhengce/zhengceku/2020-11/05/content_5557639.htm
- Monto AS, Koopman JS, Longini IM Jr. Tecumseh study of illness. XIII. Influenza infection and disease, 1976-1981. Am J Epidemiol 1985;121:811-22. [Crossref] [PubMed]
- Fraaij PL, Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine 2011;29:7524-8. [Crossref] [PubMed]
- Cowling BJ, Perera RA, Fang VJ, et al. Incidence of influenza virus infections in children in Hong Kong in a 3-year randomized placebo-controlled vaccine study, 2009-2012. Clin Infect Dis 2014;59:517-24. [Crossref] [PubMed]
- Expert consensus on diagnosis and treatment of influenza in children (2015 Edition). Chin J Appl Clin Pediatr 2015;30:1296-303.
- Yang J, Jit M, Leung KS, et al. The economic burden of influenza-associated outpatient visits and hospitalizations in China: a retrospective survey. Infect Dis Poverty 2015;4:44. [Crossref] [PubMed]
- Chiu SS, Chan KH, So LY, et al. The population based socioeconomic burden of pediatric influenza-associated hospitalization in Hong Kong. Vaccine 2012;30:1895-900. [Crossref] [PubMed]
- Shie JJ, Fang JM. Development of effective anti-influenza drugs: congeners and conjugates - a review. J Biomed Sci 2019;26:84. [Crossref] [PubMed]
- Committee on Infectious Diseases. Recommendations for Prevention and Control of Influenza in Children, 2020-2021. Pediatrics 2020;146:e2020024588. [Crossref] [PubMed]
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009;360:953-6. [Crossref] [PubMed]
- Baranovich T, Saito R, Suzuki Y, et al. Emergence of H274Y oseltamivir-resistant A(H1N1) influenza viruses in Japan during the 2008-2009 season. J Clin Virol 2010;47:23-8. [Crossref] [PubMed]
- Hama R, Jones M, Okushima H, et al. Oseltamivir and early deterioration leading to death: a proportional mortality study for 2009A/H1N1 influenza. Int J Risk Saf Med 2011;23:201-15. [Crossref] [PubMed]
- Ohkusa Y, Sugawara T, Taniguchi K, et al. Inquiry into some gap among oseltamivir use and severe abnormal behavior in Japanese children and adolescents with influenza. Drug Discov Ther 2018;12:381-3. [Crossref] [PubMed]
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis 2019;68:895-902. [Crossref] [PubMed]
- Sun PH. The latest study on traditional Chinese herbs against virus Chin Arch Tradit Chin Med 2006;4:733-5. (in Chinese).
- Huang J, He F, Bai Y, et al. Pharmacodynamics Study on Qinxiang Qingjie Oral Solution Evaluation and Analysis of Drug-Use in Hospitals of China 2017;17:1078-82. (in Chinese).
- Huang J, He F, Bai Y, et al. Study on Acute and Long-term Toxicity of Qinxiangqingjie Oral Solution Evaluation and Analysis of Drug-Use in Hospitals of China 2017;17:934-7. (in Chinese).
- Li BH, Li ZY, Liu MM, et al. Progress in Traditional Chinese Medicine Against Respiratory Viruses: A Review. Front Pharmacol 2021;12:743623. [Crossref] [PubMed]
- Li J, Ma R, Hu S, et al. Multi-center clinical trial of Qinxing Qingjie Oral Liquid treating on acute upper respiratory infection children patient with syndrome of heat in both exterior and interior China J Tradit Chin Med Pharm 2015;30:3794-6. (in Chinese).
- Hu S, Ma R, Chen X, et al. Multi-center phase III clinical trial of Qinxiang Qingjie Oral Liquid for treating pediatric patients of acute upper respiratory infection with syndrome of heat in both exterior and interior Chin J New Drugs 2017;26:1152-6. (in Chinese).
- Wang C, Shi T. Clinical study on Qinxiang Qingjie Oral Liquid combined with ribavirin in treatment of upper respiratory tract infection in children Drugs Clin 2019;34:370-3. (in Chinese).
- ICH [Internet]. Integrated addendum to ICH E6 (R1): Guideline for good clinical practice E6 (R2) 2016. [cited 2022 May 1]. Available online: https://www.cde.org.cn/ichWeb/guideIch/toGuideIch/3/0
- National Health Commission of the People’s Republic of China. Guidelines for Diagnosis and Treatment of Influenza 2018 [Internet]. [cited 2021 July 1].
- Zheng X. Guiding principles for clinical research of new drugs of traditional Chinese medicine (in Chinese). Beijing: Beijing China Medical Science and Technology Publishing House, 2002.
- Fischer JB, Prasad PA, Coffin SE, et al. Canadian Acute Respiratory Illness and Flu Scale (CARIFS) for clinical detection of influenza in children. Clin Pediatr (Phila) 2014;53:1174-80. [Crossref] [PubMed]
- Xu T, Hu S, Jin L, et al. Revision and evaluation of the reliability and validity of the Chinese version of Canadian Acute Respiratory Illness and Flu Scale Chinese Journal of Evidence-Based Pediatrics 2014;9:1-5. (in Chinese).
- Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001;20:127-33. [Crossref] [PubMed]
- Kiyohara H, Ichino C, Kawamura Y, et al. Patchouli alcohol: in vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth. J Nat Med 2012;66:55-61. [Crossref] [PubMed]
- Wu XL, Ju DH, Chen J, et al. Immunologic mechanism of Patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway in vitro. Curr Microbiol 2013;67:431-6. [Crossref] [PubMed]
- Liu F, Cao W, Deng C, et al. Polyphenolic glycosides isolated from Pogostemon cablin (Blanco) Benth. as novel influenza neuraminidase inhibitors. Chem Cent J 2016;10:51. [Crossref] [PubMed]
- Zhi HJ, Zhu HY, Zhang YY, et al. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019;57:105-16. [Crossref] [PubMed]
- Ji S, Li R, Wang Q, et al. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J Ethnopharmacol 2015;176:475-84. [Crossref] [PubMed]
- Chen L, Dou J, Su Z, et al. Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res 2011;91:314-20. [Crossref] [PubMed]
- Cheung DW, Koon CM, Wat E, et al. A herbal formula containing roots of Salvia miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages through inhibition of nuclear factor κB (NFκB) pathway. J Ethnopharmacol 2013;145:776-83. [Crossref] [PubMed]
- Xie P, Cui L, Shan Y, et al. Antithrombotic Effect and Mechanism of Radix Paeoniae Rubra. Biomed Res Int 2017;2017:9475074. [Crossref] [PubMed]
- Wang HX, Zeng MS, Ye Y, et al. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother Res 2021;35:324-36. [Crossref] [PubMed]
- Wang L, Zhang RM, Liu GY, et al. Chinese herbs in treatment of influenza: a randomized, double-blind, placebo-controlled trial. Respir Med 2010;104:1362-9. [Crossref] [PubMed]
- Wang C, Cao B, Liu QQ, et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med 2011;155:217-25. [Crossref] [PubMed]
- Li ZT, Li L, Chen TT, et al. Efficacy and safety of Ban-Lan-Gen granules in the treatment of seasonal influenza: study protocol for a randomized controlled trial. Trials 2015;16:126. [Crossref] [PubMed]


