
Ultrasound elastography in the diagnosis of biliary atresia in pediatric surgery: a systematic review and meta-analysis of diagnostic test
Introduction
Biliary atresia (BA) is a common and serious hepatobiliary disease in neonates. It is generally caused by intrahepatic BA, resulting in intrahepatic cholestasis, which further leads to liver injury and fibrosis progression from the frontal day (1). Untreated treatment often leads to liver failure and ultimately death. The clinical symptoms of BA are often similar to those of some cholestasis disorders (2-4). This brings great challenges to the clinical diagnosis of BA. Early and effective treatment of BA can significantly improve the prognosis of patients (5). Clinical study shows that the jaundice clearance rate of children with BA is 52.5% at 1 month after birth, 42.2% at 2 months after surgery, and only 10.2% at 73 days after surgery (6). Other studies have shown that for children with BA, open surgery can provide the optimal survival time of their own liver within 55 days after birth (7-9). If the child was older than 62 days, each 9-day delay reduced the surgical success rate by 48.0% and the risk of liver failure obviously increased. As the liver fibrosis process of BA is earlier and faster than other biliary diseases, the early differential diagnosis of BA is very important. However, due to the clinical characteristics of BA similar to other biliary diseases, the early diagnosis of BA is a major clinical challenge (10-12).
In recent years, ultrasound elastography (USE) has been widely used in adult liver, breast, thyroid, prostate, and other organs, and has shown broad application prospects in non-invasive evaluation of tissue stiffness. USE is a newly developed elastography technology based on two-dimensional ultrasound imaging (13,14). By measuring the propagation velocity of shear wave in tissue, the hardness of tissue can be measured indirectly and quantitatively. Compared with 1d elastography, USE is less affected by tissues and patients, and shows faster and more accurate measurements and great advantages in the diagnosis and treatment of childhood diseases (15-17). Compared with other ultrasound techniques, USE has the following advantages. First, it is based on traditional ultrasound diagnostic equipment and makes full use of real-time grayscale imaging mode to evaluate tissue morphological changes or avoid large vessels, which is superior to transient elastography (TE) (18,19). Second, the use of larger and wider s-waves enhances the ability to identify the extent of liver fibrosis, making it more effective in the diagnosis of liver fibrosis. Finally, USE allows real-time analysis and mapping of elastograms in this area, which exceeds the capabilities of TE and acoustic radiation force impulse (ARFI) (20). However, its test results are susceptible to exercise, diet, liver congestion, transaminase, inflammation, and other factors. Therefore, its diagnostic efficiency in child missile lock-in is still controversial. For example, some scholars compared the effectiveness of USE and magnetic resonance cholangiopancreatography (MRCP) in the diagnosis of neonatal BA. The results show that diagnostic accuracy of USE is lower than that of MRCP. Therefore, the diagnostic efficacy of USE in pediatric BA needs further study and unification (21,22). This work intended to conduct a meta-analysis of the related literature on USE in diagnosing BA in recent years, and preliminatively evaluate its diagnostic value. We provide the following articles based on the PRISMA-DTA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-159/rc).
Methods
Literature retrieval
Articles published from establishment date till now were searched from PubMed, Medline, Embase, Chinese BioMedical Literature Database (CBM), WanFang, and other databases. The subject terms included “Ultrasonic elastography” and “biliary atresia” when searching. The above phrases were combined for search to obtain more documents. The search terms appeared in the title, keywords, abstract, etc. Some references cited by the articles could be traced back.
How to include and exclude the literature
According to the principles of PICOS, the literature retrieval standards were defined. Articles meeting following criteria could be included in this study: (I) articles that were diagnostic study designs; (II) studies involving research subjects who were obstructive jaundice patients and met the obstructive jaundice diagnostic criteria; (III) articles whose test standard was USE; (IV) studies that applied the gold standard for the diagnosis of BA (i.e., clear fibrosis of the gallbladder cord by surgical exploration, intraoperative cholangiography suggesting that the intra- and extra-hepatic bile ducts were not developed, and postoperative pathological examination confirmation of BA); (V) studies that applied the gold standard for diagnosis of non-BA (i.e., intraoperative cholangiography indicating patency of the bile ducts inside and outside the liver, pathological examination of liver biopsy indicating other cholestatic diseases, and clinical conservative treatment and follow-up of jaundice resolution); (VI) articles involving observation indicators such as sensitivity and specificity, and those in which the data required by the four-grid table could be extracted. Articles meeting the following criteria were excluded: (I) reviews, conference papers, dissertations, newsletters, case reports, etc.; (II) republications or duplicates of published data; and (III) articles that did not include the data required by the four-grid table.
Quality assessment
Two researchers were required to use RevMan5.3 software (Cochrane Collaboration, UK) and the quality assessment of diagnostic accuracy studies (QUADAS) for quality assessment of the searched articles. If there was any disagreement between the two researchers, a third researcher was invited to conduct an intervention evaluation, and a consensus could be reached through discussion. The evaluation criteria covered the cases selection, assessment method, gold standard, assessment process, and progress.
A total of 16 items were included in the QUADAS tool to evaluate the included articles, and the quality assessment results were displayed as “Yes”, “No”, and “Unclear”. “Yes” signified that the standard was met and “No” meant that the standard was not satisfied. When the information was incomprehensive or only partially met the standard, it was defined as “Unclear”.
Data extraction
Two researchers were required to independently read the titles and abstracts of the retrieved literature, complete the literature selection process, and obtain the full texts, and extract required information. The two researchers could discuss to solve the disagreement, and a third researcher was required to arbitrate in cases where a unanimous opinion could not be reached.
The data to be extracted included first author, year of publication, research country, detection method, gold standard, numbers of true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN), and other information. The researchers were required to verify the data and then conduct a systematic analysis.
Statistical analysis
RevMan5.3 software was adopted for risk bias analysis. The SROC curve was adopted for diagnostic analysis; a shoulder-like shape of SROC curve meant the consistency was found, or the sensitivity was negatively correlated with specificity (P<0.05). After SROC curve was obtained, the area under SROC curve (AUC) should be calculated, and the value of the diagnostic test based on the AUC was calculated. An AUC of 0.5–0.7, 0.7–0.9, and 0.9–1.0 signified a low, medium, and high diagnosis rate, respectively.
Results
Literature retrieval and basic characteristics of the included articles
A total of 864 records were initially retrieved, and 837 abstracts related to this study were obtained after deleting duplicates. After the titles and abstracts of these articles were read, they initially screened and retrieved 48 articles were qualified. After further reading the full texts of these studies and excluding non-random, duplicate publications, and unavailable articles, seven qualified articles were finally included (23-29). The literature retrieval process was displayed in Figure 1, and related basic information was given in Table 1.
Table 1
| First author | Year of publication | TP | FP | FN | TN | Gold standard |
|---|---|---|---|---|---|---|
| Duan | 2019 | 37 | 0 | 14 | 87 | Cholangiography |
| Wu | 2018 | 15 | 0 | 0 | 33 | Cholangiography |
| Hanquinet | 2015 | 7 | 1 | 3 | 9 | Cholangiography |
| Shima | 2012 | 7 | 0 | 0 | 1 | Cholangiography |
| Wang | 2016 | 37 | 1 | 0 | 31 | Cholangiography |
| Zhou | 2017 | 109 | 12 | 11 | 40 | Cholangiography |
| Dillman | 2019 | 13 | 0 | 0 | 28 | Cholangiography |
TP, true positives; FP, false positives; TN, true negatives; FN, false negatives.
Quality assessment
Firstly, the tool recommended by the Cochrane System Review Manual was adopted for quality assessment. The results were illustrated in Figures 2,3. The evaluation revealed that Dillman et al.’s equivalence (29) was not clear about the risk, and Duan et al. (23) was high-risk. Taken together, the seven included articles exhibited a large proportion of “low risk” and “low concerns”, which suggested that the included studies met the analysis requirements.
Subsequently, the QUADAS was employed for quality assessment each article (Table 2). This evaluation showed that all seven articles showed a low risk of bias, satisfying the requirements.
Table 2
| First author | Year of publication | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duan | 2019 | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Wu | 2018 | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Hanquinet | 2015 | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Shima | 2012 | Y | Y | Y | U | Y | Y | Y | Y | Y | U | U | Y | Y | Y |
| Wang | 2016 | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Zhou | 2017 | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Dillman | 2019 | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
1–14 were the criteria of the QUADAS items. Y, Yes; U, Unclear.
Meta-analysis of BA diagnosed with USE
We evaluated the diagnostic value of USE in BA in pediatric surgery, and the results are shown in Figure 4. Our findings indicated that the estimated sensitivity range of USE for the diagnosis of BA in pediatric surgery was 0.72–1.00, and the specificity of diagnosis was 0.74–1.00.
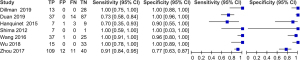
SROC of BA diagnosed by USE
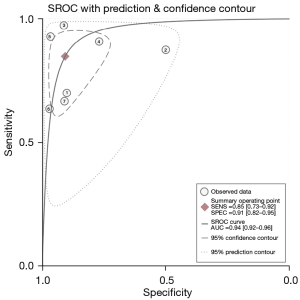
A bivariate model was used to analyze the diagnostic value of USE in BA in pediatric surgery (as illustrated in Figure 5). Our findings suggested that aggregate estimated value of sensitivity and specificity for different studies were 0.93 (95% CI: 0.70–1.00) and 0.95 (95% CI: 0.35–1.00), respectively, and the AUC was 0.93.
Discussion
Elastography technology is a new ultrasound imaging technology that has been gradually developed over the past 20 years. It can detect the propagation velocity of shear waves in tissues and quantitatively measure tissue hardness, and is commonly used in liver hardness determination in clinical practice (30). Its earliest clinical application is the one-dimensional TE, namely Fibroscan, but this is subject to the interference of the tissue itself. In addition, even slight movement by the patient during the detection process will interfere with the final detection result. Moreover, there are particular disadvantages in children with greater cooperation difficulties (especially newborns) (31).
In recent years, with the advancement of elastography technology, two-dimensional real-time shear wave elastography has slowly been adopted in clinical practice. It can select the region of interest (ROI) based on conventional two-dimensional ultrasound imaging. ARFI technology is used to generate shear waves inside the tissue, which are then received by the same ultrasonic probe to measure the wave and velocity of the shear wave (32,33). This is the most advanced elastography technology in the world. Numerous studies have shown that TE test results exhibit a good correlation with the pathological gold standard liver fibrosis classification (34,35). It is an accurate and non-invasive method for assessing the degree of liver cirrhosis; its diagnostic sensitivity can reach 80% in children and adolescents (95% CI: 70–87%), and its specificity can reach 90% (95% CI: 84–96%) (36).
In addition, multiple studies have shown that the elastic stiffness of patients with BA is also strongly correlated with the classification of pathological liver fibrosis (37,38). Due to intrahepatic and extrahepatic bile duct obstruction caused by intrahepatic cholestasis, the liver fibrosis of BA progresses faster, and the liver stiffness value is markedly different to that of infant hepatitis syndrome and other diseases (39). Therefore, the preoperative measurement of liver stiffness may be helpful for diagnosis and treatment (40).
USE technology in the diagnosis of BA is in its infancy. Most case-control studies have small sample sizes and generally of low quality. Therefore, comprehensive analysis is required according to specific rules. All BA studies that the gold standard for diagnosing BA is not uniform, and the final diagnosis of BA mostly depends on clinical follow-up (41). At the same time, with the exception of a few studies, most research does not explain the time interval between the examination to be tested and the implementation of the gold standard, which is a major flaw of previous studies.
According to the results of various studies and the combined results of meta-analysis, USE has a certain value in the diagnosis of BA, and its sensitivity and specificity are around 90%. Ultrasound is a highly subjective examination that is closely related to the experience of the examiner, while USE is a quantitative measurement of tissue stiffness based on two-dimensional ultrasound. In the future, the addition of elasticity measurement technology to ultrasound examination will provide new insights in the diagnosis of BA.
USE is the latest reliable, non-invasive method to assess the degree of liver fibrosis. There are a limited number of studies on BA, and further research is needed to evaluate the prognosis of BA. Seven articles were included according to the inclusion and exclusion criteria. The results suggested that the fitting AUC of USE for diagnosis of BA was 0.93, the sensitivity was 0.93 (95% CI: 0.72–1.00), and the specificity was 0.95 (95% CI: 0.74–1.00). The diagnostic efficiency fluctuates greatly, which may be affected by manipulation and image interpretation experience of doctors.
Conclusions
It aimed to evaluate systematically the clinical application value of USE in diagnosing BA. Seven related articles were included, and meta-analysis results of diagnostic accuracy rate showed that the sensitivity and specificity of USE in the diagnosis of BA were 0.93 (95% CI: 0.72–1.00) and 0.95 (95% CI: 0.74–1.00), respectively, and the AUC was 0.93. These results indicate that this method has moderate efficiency for the diagnosis of ischemic cerebrovascular disease.
However, we only used computer retrieval methods to search for relevant articles, and did not consider evaluating the effect of combining USE and other imaging techniques in the diagnosis of BA. This would have resulted in a larger number of documents, and the article quality could have been controlled according to standards outlined in this paper.
In summary, the results could offer a theoretical basis for promoting and applying clinical diagnosis of BA via USE. We found that USE provided a certain value in BA diagnosis. However, there was a lack of high-quality prospective clinical diagnostic tests included in this analysis; hence, a prospective study with more samples was needed to confirm the accuracy of USE in the diagnosis of BA, and to further unify and improve the detection methods of USE in children.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-159/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-159/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hwang J, Yoon HM, Kim KM, et al. Assessment of native liver fibrosis using ultrasound elastography and serological fibrosis indices in children with biliary atresia after the Kasai procedure. Acta Radiol 2021;62:1088-96. [Crossref] [PubMed]
- Lendahl U, Lui VCH, Chung PHY, et al. Biliary Atresia - emerging diagnostic and therapy opportunities. EBioMedicine 2021;74:103689. [Crossref] [PubMed]
- Sandberg JK, Sun Y, Ju Z, et al. Ultrasound shear wave elastography: does it add value to gray-scale ultrasound imaging in differentiating biliary atresia from other causes of neonatal jaundice? Pediatr Radiol 2021;51:1654-66. [Crossref] [PubMed]
- Hertel PM, Estes MK. Rotavirus and biliary atresia: can causation be proven? Curr Opin Gastroenterol 2012;28:10-7. [Crossref] [PubMed]
- Caruso M, Cuocolo R, Di Dato F, et al. Ultrasound, shear-wave elastography, and magnetic resonance imaging in native liver survivor patients with biliary atresia after Kasai portoenterostomy: correlation with medical outcome after treatment. Acta Radiol 2020;61:1300-8. [Crossref] [PubMed]
- Sokol RJ, Mack C. Etiopathogenesis of biliary atresia. Semin Liver Dis 2001;21:517-24. [Crossref] [PubMed]
- Shin NY, Kim MJ, Lee MJ, et al. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med 2014;33:853-64. [Crossref] [PubMed]
- Wang G, Chen H, Xie X, et al. 2D shear wave elastography combined with age and serum biomarkers prior to kasai surgery predicts native liver survival of biliary atresia infants. J Intern Med 2020;288:570-80. [Crossref] [PubMed]
- Thumar V, Squires JH, Spicer PJ, et al. Ultrasound Elastography Applications in Pediatrics. Ultrasound Q 2018;34:199-205. [Crossref] [PubMed]
- Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017;7:1303-29. [Crossref] [PubMed]
- Yoon H, Shin HJ, Kim MJ, et al. Quantitative Imaging in Pediatric Hepatobiliary Disease. Korean J Radiol 2019;20:1342-57. [Crossref] [PubMed]
- Mazur R, Celmer M, Silicki J, et al. Clinical applications of spleen ultrasound elastography - a review. J Ultrason 2018;18:37-41. [Crossref] [PubMed]
- Honsawek S, Chongsrisawat V, Praianantathavorn K, et al. Elevation of serum galectin-3 and liver stiffness measured by transient elastography in biliary atresia. Eur J Pediatr Surg 2011;21:250-4. [Crossref] [PubMed]
- Shen QL, Chen YJ, Wang ZM, et al. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. World J Gastroenterol 2015;21:6931-6. [Crossref] [PubMed]
- Liu YF, Ni XW, Pan Y, et al. Comparison of the diagnostic value of virtual touch tissue quantification and virtual touch tissue imaging quantification in infants with bili-ary atresia. Int J Clin Pract 2021;75:e13860. [Crossref] [PubMed]
- Sohn H, Park S, Kang Y, et al. Predicting variceal bleeding in patients with bili-ary atresia. Scand J Gastroenterol 2019;54:1385-90. [Crossref] [PubMed]
- Zhou W, Li X, Zhang N, et al. The combination of conventional ultrasound and shear-wave elastography in evaluating the segmental heterogeneity of liver fibrosis in biliary atresia patients after Kasai portoenterostomy. Pediatr Surg Int 2021;37:1099-108. [Crossref] [PubMed]
- Honsawek S, Chayanupatkul M, Chongsrisawat V, et al. Increased osteopontin and liver stiffness measurement by transient elastography in biliary atresia. World J Gastroenterol 2010;16:5467-73. [Crossref] [PubMed]
- Yan H, Du L, Zhou J, et al. Diagnostic performance and prognostic value of elastography in patients with biliary atresia and after hepatic portoenterostomy: proto-col for a systematic review and meta-analysis. BMJ Open 2021;11:e042129. [Crossref] [PubMed]
- Chen Y, Zhao D, Gu S, et al. Three-color risk stratification for improving the di-agnostic accuracy for biliary atresia. Eur Radiol 2020;30:3852-61. [Crossref] [PubMed]
- Uchida H, Sakamoto S, Kobayashi M, et al. The degree of spleen stiffness meas-ured on acoustic radiation force impulse elastography predicts the severity of portal hypertension in patients with biliary atresia after portoenterostomy. J Pediatr Surg 2015;50:559-64. [Crossref] [PubMed]
- Sirisomboonlarp K, Udomsinprasert W, McConachie E, et al. Increased serum glypican-3 is associated with liver stiffness and hepatic dysfunction in children with biliary atresia. Clin Exp Hepatol 2019;5:48-54. [Crossref] [PubMed]
- Duan X, Peng Y, Liu W, et al. Does Supersonic Shear Wave Elastography Help Differentiate Biliary Atresia from Other Causes of Cholestatic Hepatitis in Infants Less than 90 Days Old? Compared with Grey-Scale US. Biomed Res Int 2019;2019:9036362. [Crossref] [PubMed]
- Wu JF, Lee CS, Lin WH, et al. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology 2018;68:616-24. [Crossref] [PubMed]
- Hanquinet S, Courvoisier DS, Rougemont AL, et al. Contribution of acoustic ra-diation force impulse (ARFI) elastography to the ultrasound diagnosis of biliary atresia. Pediatr Radiol 2015;45:1489-95. [Crossref] [PubMed]
- Shima H, Igarashi G, Wakisaka M, et al. Noninvasive acoustic radiation force impulse (ARFI) elastography for assessing the severity of fibrosis in the post-operative patients with biliary atresia. Pediatr Surg Int 2012;28:869-72. [Crossref] [PubMed]
- Wang X, Qian L, Jia L, et al. Utility of Shear Wave Elastography for Differenti-ating Biliary Atresia From Infantile Hepatitis Syndrome. J Ultrasound Med 2016;35:1475-9. [Crossref] [PubMed]
- Zhou LY, Jiang H, Shan QY, et al. Liver stiffness measurements with supersonic shear wave elastography in the diagnosis of biliary atresia: a comparative study with grey-scale US. Eur Radiol 2017;27:3474-84. [Crossref] [PubMed]
- Dillman JR, DiPaola FW, Smith SJ, et al. Prospective Assessment of Ultrasound Shear Wave Elastography for Discriminating Biliary Atresia from other Causes of Neo-natal Cholestasis. J Pediatr 2019;212:60-65.e3. [Crossref] [PubMed]
- Chen S, Liao B, Zhong Z, et al. Supersonic shearwave elastography in the as-sessment of liver fibrosis for postoperative patients with biliary atresia. Sci Rep 2016;6:31057. [Crossref] [PubMed]
- Napolitano M, Franchi-Abella S, Damasio BM, et al. Practical approach for the diagnosis of biliary atresia on imaging, part 2: magnetic resonance cholecystopancrea-tography, hepatobiliary scintigraphy, percutaneous cholecysto-cholangiography, endo-scopic retrograde cholangiopancreatography, percutaneous liver biopsy, risk scores and decisional flowchart. Pediatr Radiol 2021;51:1545-54. [Crossref] [PubMed]
- Galina P, Alexopoulou E, Mentessidou A, et al. Diagnostic accuracy of two-dimensional shear wave elastography in detecting hepatic fibrosis in children with autoimmune hepatitis, biliary atresia and other chronic liver diseases. Pediatr Radiol 2021;51:1358-68. [Crossref] [PubMed]
- Leschied JR, Dillman JR, Bilhartz J, et al. Shear wave elastography helps dif-ferentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr Radiol 2015;45:366-75. [Crossref] [PubMed]
- Zhou K, Xie G, Wen J, et al. Histamine is correlated with liver fibrosis in biliary atresia. Dig Liver Dis 2016;48:921-6. [Crossref] [PubMed]
- Sun S, Xu M, Zhuang P, et al. Effect and mechanism of vitamin D activation disorder on liver fibrosis in biliary atresia. Sci Rep 2021;11:19883. [Crossref] [PubMed]
- Kilgore A, Mack CL. Update on investigations pertaining to the pathogenesis of biliary atresia. Pediatr Surg Int 2017;33:1233-41. [Crossref] [PubMed]
- Wang J, Xu Y, Chen Z, et al. Liver Immune Profiling Reveals Pathogenesis and Therapeutics for Biliary Atresia. Cell 2020;183:1867-1883.e26. [Crossref] [PubMed]
- Udomsinprasert W, Angkathunyakul N, Jittikoon J, et al. Cartilage oligomeric matrix protein as a marker of progressive liver fibrosis in biliary atresia. Sci Rep 2021;11:16695. [Crossref] [PubMed]
- Samyn M. Transitional care of biliary atresia. Semin Pediatr Surg 2020;29:150948. [Crossref] [PubMed]
- Kim YY, Kim MJ, Shin HJ, et al. Interconversion of elasticity measurements between two-dimensional shear wave elastography and transient elastography. Med Ultrason 2018;20:127-33. [Crossref] [PubMed]
- Lee S, Kim MJ, Lee MJ, et al. Hepatic subcapsular or capsular flow in biliary atresia: is it useful imaging feature after the Kasai operation? Eur Radiol 2020;30:3161-7. [Crossref] [PubMed]





