
The relationship between vascular endothelial growth factor expression and the risk of childhood nephroblastoma: systematic review and meta-analysis
Introduction
Blastomas are malignant solid tumors that most commonly occur in childhood. Nephroblastoma, also known as Wilms tumor (WT), is an embryonic tumor originating from undifferentiated mesodermal tissue (1). WT mainly occurs in infants, accounting for about 95% of childhood kidney tumors, and the incidence of nephroblastoma in infants is about 1/1,000,000 (2-4). Adult Wilms tumor (AWT) is a clinically rare malignant kidney tumor, with an incidence of less than 3%. AWT has a high degree of malignancy, rapid growth, early metastasis, difficult preoperative diagnosis, and high misdiagnosis rate (5). Tumorigenesis may involve WT1 (Wilms Tumor suppressor gene), WT2, P53 and other genes, and may also be related to congenital genetic factors.
At present, the etiology of nephroblastoma is not clear and may be related to gene mutations that regulate normal embryonic development of the genitourinary tract (6). Most patients have palpable abdominal mass as the initial symptom, while some patients have hematuria, fever, urinary tract infection, varicocele, hypertension or hypotension, anemia, and other symptoms. At present, treatment of nephroblastoma involves multidisciplinary combination therapy, including surgery, chemotherapy, radiotherapy, and targeted therapy, with an overall cure rate of about 90%.
The growth and development of a tumor requires rapid angiogenesis, while the growth of blood vessels in nontumor sites is slow or even nonproliferating. Inhibition of angiogenesis can significantly prevent the development, spread, and metastasis of tumor tissue (7). Vascular endothelial growth factor (VEGF) is the most commonly used target for antiangiogenic therapy (8). VEGF is known to induce angiogenesis, and anti-angiogenesis therapy targeting VEGF receptor (VEGFR) is widely used in cancer treatment. VEGF is the most thoroughly studied factor inducing endothelial cell proliferation and angiogenesis. VEGF expression in serum and tissues of nephroblastoma is associated with poor prognosis, which lays a theoretical foundation for anti-angiogenic therapy (9). VEGF regulates angiogenesis and vascular permeability by activating 2 receptors, VEGFR-1, and VEGFR-2. Apatinib is a small molecule antiangiogenic agent that selectively binds to inhibit VEGFR-2 kinase activity, which reduces VEGF-mediated tumor endothelial cell migration and proliferation, thereby reducing tumor microtubule density and inhibiting the growth of nephroblastoma (10). Currently, bevacizumab, AZD2171, and other VEGF/VEGFR pathway inhibitors are being marketed or are in clinical trials (11,12).
Some studies have applied whole-macrophage inhibitors, such as colony stimulating factor signaling pathway inhibitors, which has become a new direction of targeted therapy. Some drugs that specifically inhibit macrophages in tumor microenvironment have been used to enhance the anti-tumor effect. In this paper, the expression and correlation of VEGF in nephroblastoma were studied, hoping to provide theoretical basis for targeted therapy for refractory and recurrent pediatric nephroblastoma. To fully understand the relationship between VEGF expression and pediatric nephroblastoma, this study conducted a meta-analysis to systematically evaluate literature published in domestic journals on the use of immunohistochemistry to detect the relationship between VEGF expression and pediatric nephroblastoma. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-593/rc).
Methods
Search strategy
Independent RCTs studies data on the expression and significance of VEGF in pediatric nephroblastoma were collected by searching PubMed, Cochrane Library, Web of Science, CNKI, CBM, et al. The search term was “corridor tube endothelial growth factor, VEGF, nephroblastoma” and there was no limit on language.
Inclusion criteria
The inclusion criteria were: independent RCTs studies on the expression and significance of VEGF in pediatric nephroblastoma; pediatric nephroblastoma case group confirmed by pathological examination and control group with normal renal tissue; studies with similar problems and methods; and studies providing odds ratio (OR) and 95% confidence interval (CI), or able to be converted into OR and 95% CI. Inclusion criteria for inclusion studies should be clarified using PICOS criteria. PICOS criterion was used to screen and include: P: Participant or Patient, study object; I: Intervention; C: Comparison; O: Outcome; S: Study design.
Exclusion criteria
Studies were excluded according to the following criteria: no control group, immunohistochemistry not used for detection, and literature with too little information to be useful.
Literature quality assessment
The methodological quality of each of the included randomized control trials (RCTs) was evaluated using the method recommended by the Cochrane Handbook for Systematic Reviews of Interventions version 5.1. The evaluation considered whether: (I) randomization was adequate; (II) concealed allocation was used; (III) blinding was implemented; (IV) the loss of follow-up and withdrawal was reported; (V) the baseline was comparable. For concealed allocation, experiments were rated A (completely concealed), B (unclear if concealed), C (inadequate concealment), and D (no concealment used). All other indicators were rated A (yes), B (unclear), or C (no). If all evaluation items were graded A, the study had a low degree of bias, with the lowest possibility of the occurrence of all types of bias, and the quality was rated A. If 1 or more items were B, the test had a medium probability of corresponding bias, and the quality was rated as B. If 1 or more of the items was C, the test had a high probability of corresponding bias, and the quality was rated as C (Figure 1).
Bias analysis
The Cochrane Handbook’s bias risk assessment tool was used by 2 independent researchers to assess the included studies for bias. The studies were assessed on the basis of random assignment, allocation concealment, blinded researchers and participants, blinded measurement results, data integrity, selective reports in 7 aspects, and other biases. Risk was evaluated as “low risk”, “high risk”, and “not clear”. If there was ambiguity concerning the evaluation results, a third researcher was invited to discuss the decision. The literature publication bias of this study was analyzed by funnel plot, as shown in the figure below. Most of the included literatures were within the triangle area, suggesting that the publication bias of this study was not obvious. The literature included in this study was a randomized controlled study, which was analyzed according to the Cochrane RoB 2.0 principle (Figure 2).
Statistical analysis
In accordance with meta-analysis requirements, the analysis process involved literature collection and evaluation, quantitative data merger, and results evaluation and interpretation. The index for measuring risk factors was OR value, and a consistency test of the data was conducted. The fixed effects model was used when the difference was not statistically significant, otherwise the random effect model was adopted. Statistical analyses were completed with RevMan 4.2.2 software. P<0.05 was considered a statistically significant difference.
Results
Basic information of the included literature
Based on the inclusion and exclusion criteria, 12 studies were included in the meta-analysis (13-24). The results of each study were tested for consistency (P=0.29) and a fixed effects model analysis was used (Table 1).
Table 1
| Study | Age (SD), years | Gender (male/female) | SOFA score (SD), 6.3–6.7 | APACHE II (SD), 20.1–22.5 | Outcome | |
|---|---|---|---|---|---|---|
| Experimental group | Control group | |||||
| Maslak et al., 2018 | 4.2±1.02 | 40/45 | NA | 95.4–96.6 | VEGF (+) | VEGF (−) |
| Chuk et al., 2018 | 4.1±1.14 | 42/34 | 8.2–10.2 | 22.4–24.5 | VEGF (+) | VEGF (−) |
| Rivera et al., 2020 | 8.2±1.05 | 57/47 | 5.5–6.5 | NA | VEGF (+) | VEGF (−) |
| Chagaluka et al., 2020 | 5.2±1.50 | 73/38 | NA | 100.2–107.5 | VEGF (+) | VEGF (−) |
| Tang et al., 2020 | 3.5±1.18 | 24/20 | NA | NA | VEGF (+) | VEGF (−) |
| Yin et al., 2019 | 4.4±1.70 | NA/NA | NA | NA | VEGF (+) | VEGF (−) |
| Ehrlich et al., 2020 | 5.5±2.02 | 52/27 | 9.4–10.9 | 23.2–24.8 | VEGF (+) | VEGF (−) |
| Lin et al., 2019 | 8.6±1.45 | 62/25 | 10.8–11.2 | NA | VEGF (+) | VEGF (−) |
| Rautenberg et al., 2019 | 8.9±2.05 | 108/96 | NA | NA | VEGF (+) | VEGF (−) |
| Gavens et al., 2020 | 4.5±1.60 | 49/51 | 9.2–9.6 | NA | VEGF (+) | VEGF (−) |
| Koshinaga et al., 2019 | 5.2±1.55 | 59/68 | 7.1–8.5 | 23.5–24.9 | VEGF (+) | VEGF (−) |
| Irtan et al., 2019 | 3.2±1.25 | 74/88 | NA | NA | VEGF (+) | VEGF (−) |
NA, not applicable; SOFA, sequential organ failure assessment; APACHE II, Acute Physiology and Chronic Health Evaluation II; SD, standard deviation.
Results of literature screening
A total of 350 documents were initially retrieved using computer search databases PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang, and so on. After strict screening according to the inclusion and exclusion criteria, 12 documents with a total of 1,226 participants were finally included in the study (13-24). The literature screening flow chart is shown in Figure 3.
Meta-analysis of the included literature
The 12 literatures reported the expression of VEGF in childhood nephroblastoma. Positive expression of VEGF showed a statistically significant difference between the case group and the control group (OR =9.06, 95% CI: 6.97–11.76, P<0.00001). There was acceptable heterogeneity between studies (I2=32%, Z=16.49; Figure 4).
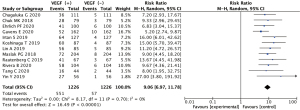
VEGF expression in nephroblastoma of different tissue types
The 12 literatures reported the expression of VEGF in different tissue types of nephroblastoma, including favorable histology (FH) type and unfavorable histology (UH) type. There was a statistically significant difference in positive expression of VEGF between the FH group and the UH group (OR =1.17, 95% CI: 1.07–1.27, P=0.0006), and there was acceptable heterogeneity between studies (I2=0%, Z=3.45; Figure 5).
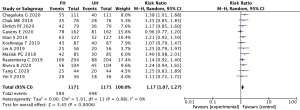
VEGF expression in nephroblastoma at different clinical stages
The 12 literatures reported the expression of VEGF at different clinical stages of nephroblastoma (including stage I–II and III–IV). Positive expression of VEGF showed a statistically significant difference between the stage I–II group and the stage III–IV group (OR =0.49, 95% CI: 0.42–0.58, P<0.00001). There was acceptable heterogeneity between studies (I2=0%, Z=8.25; Figure 6).
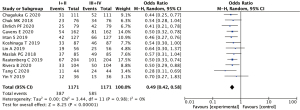
VEGF expression in nephroblastoma with or without tumor metastasis
The 12 literatures reported the expression of VEGF in nephroblastoma with and without tumor metastasis. Positive expression of VEGF showed no statistically significant difference between the group with and without tumor metastasis (OR =1.08, 95% CI: 0.86–1.36, P=0.50). There was acceptable heterogeneity between studies (I2=0%, Z=0.68; Figure 7).
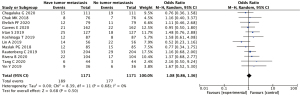
Discussion
At present, VEGF is the most effective angiogenesis-stimulating factor known (25). VECF is an important factor for stimulating the proliferation and migration of vascular endothelial cells and can affect vascular permeability. Normal tissues can also produce a small amount of VEGF to regulate the proliferation of endodermal cells in their tissues. However, the expression of VEGF in cancer cells is significantly higher than that in adjacent tissues, resulting in vascular injury and invasion during cancer metastasis, often accompanied by platelet activation and aggregation (26). VEGF changes the formation process of gene activation in endothelial cells, upregulates the expression of urokinase plasminogen activator (uPA), tissue plasminogen activator (tPA), and plasminogen activator inhibitor 1 (PAI-1), and induces the expression of proteolytic enzyme, interstitial collagenase, and tissue factor in endothelial cells, that is, induces vascular formation. High expression of VEGF messenger RNA (mRNA) and protein has been found in malignant tumor tissues and in vitro cultured tumor cell lines, including gastric cancer, colon cancer, liver cancer, lung cancer, breast cancer, glioma, bladder cancer, and kidney cancer (27). The correlation between VEGF and malignant tumor has been reported in adults but rarely in children (28). Nephroblastoma is the most common malignant tumor of the urinary system, accounting for 8% of solid tumors in children, and is still an important disease threatening children's health (29).
Wiles et al. (30) first found that there was a special vascular distribution phenomenon in the tumor tissues of a mouse model of nephroblastoma, including vascular structure disorder, vascular plexus, microaneurysm formation, and local hemorrhage, which was significantly different from the orderly vascular distribution in normal tissues. Since then, Hisada et al. (31) confirmed that this pathological vascular structure in childhood nephroblastoma was related to the upregulation of VEGF expression level. Zhang et al. (32) found that the expression level of VEGF in children with nephroblastoma was significantly higher than that in children without nephroblastoma. Lin et al. (33) also found that serum VEGF expression was high in children with nephroblastoma, but VEGF level decreased sharply after radical tumor resection. These results suggested that preoperative VEGF level was pathological rather than physiological, indicating that the tumor itself was the most likely source of VEGF high expression, although it may not be the only source (34). Serum VEGF level was higher in children who died within 6 months after surgery, suggesting that postoperative VEGF expression level may be an important indicator for predicting the metastasis and prognosis of nephroblastoma (35).
This study had some limitations: (I) among the 12 included studies, each index standard was different, and there were a large number of them, which inevitably leads to errors in data statistics; (II) differences in treatment regimens and corresponding methodologies between studies may have contributed to heterogeneity; (III) many of the projects included in this study were open trials, which may also have increased the risk of literature bias. Heterogeneity may result from differences and diversity in the inclusion criteria of patients in the studies, interventions, and measures across a range of studies, or from variations in the inherent authenticity of those studies. Statistical heterogeneity is used specifically to describe the degree of variation in effect sizes across a series of studies and to indicate variability between studies except for foreseeable chance.
In this study, meta-analysis was conducted to compare pediatric nephroblastoma tissues with normal adjacent renal tissues. The results (OR =9.06, 95% CI: 6.97–11.76, P<0.00001) and acceptable heterogeneity between studies (I2=0%, Z=16.49) suggested that VEGF was highly expressed in Wilms tumor tissues compared with normal tissues. This is consistent with the findings of the studies discussed above. There were significant differences in the expression of CDl63 and VEGF in the adjacent tissues and the nephroblastoma tissues (P<0.05), suggesting that TAM aggregation was highly expressed in the tumor cell region and presented a consistent trend with vascular proliferation. Many studies have shown that M2 TAM is positively correlated with tumor angiogenesis. TAM can regulate tumor vascular proliferation, thereby promoting blood vessel growth, as well as tumor cell growth and metastasis. Tumor metastasis and growth require blood supply, blood vessels.
Neovascularization is required, which also indicates the correlation between VEGF expression and the survival and prognosis of nephroblastoma.
Meta-analysis of VEGF expression and clinical stage of nephroblastoma showed a significant correlation (P<0.01), indicating that clinical stage of nephroblastoma affected the expression of VEGF. There was no significant correlation between VEGF expression and tumor metastasis (P>0.05). In conclusion, we found that there was a high expression of VEGF in childhood nephroblastoma, which was affected by clinical stage, but there was no significant correlation with tumor metastasis. The expression of VEGF plays an important role in the occurrence and development of childhood nephroblastoma and could help guide clinicians to evaluate the disease and treatment in children.
Acknowledgments
Funding: This work was funded by the Special Scientific Research Project of Orthopedics (Shangantong), Sichuan Medical Association (No. 2019SAT19).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-593/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-593/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young MD, Mitchell TJ, Vieira Braga FA, et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 2018;361:594-9. [Crossref] [PubMed]
- Awal HB, Nandula SR, Domingues CC, et al. Linagliptin, when compared to placebo, improves CD34+ve endothelial progenitor cells in type 2 diabetes subjects with chronic kidney disease taking metformin and/or insulin: a randomized controlled trial. Cardiovasc Diabetol 2020;19:72. [Crossref] [PubMed]
- Wagner KD, El Maï M, Ladomery M, et al. Altered VEGF Splicing Isoform Balance in Tumor Endothelium Involves Activation of Splicing Factors Srpk1 and Srsf1 by the Wilms' Tumor Suppressor Wt1. Cells 2019;8:41. [Crossref] [PubMed]
- Tan JJ, Guyette JP, Miki K, et al. Human iPS-derived pre-epicardial cells direct cardiomyocyte aggregation expansion and organization in vitro. Nat Commun 2021;12:4997. [Crossref] [PubMed]
- Luo Y, Liu W, Zhu Y, et al. KIF11 as a potential cancer prognostic marker promotes tumorigenesis in children with Wilms tumor. Pediatr Hematol Oncol 2022;39:145-57. [Crossref] [PubMed]
- Li LJ, Wang YL, Yuan LQ, et al. Autophagy Inhibition in Childhood Nephroblastoma and the Therapeutic Significance. Curr Cancer Drug Targets 2018;18:295-303. [Crossref] [PubMed]
- Chen B, Li WT, Wang FI. A blastema-predominant canine renal nephroblastoma with gingival metastasis: case report and literature review. J Vet Diagn Invest 2018;30:430-7. [Crossref] [PubMed]
- Li Y, Lei C, Xiang B, et al. Extrarenal teratoma with nephroblastoma in the retroperitoneum: Case report and literature review. Medicine (Baltimore) 2017;96:e8670. [Crossref] [PubMed]
- Hötker AM, Lollert A, Mazaheri Y, et al. Diffusion-weighted MRI in the assessment of nephroblastoma: results of a multi-center trial. Abdom Radiol (NY) 2020;45:3202-12. [Crossref] [PubMed]
- Corbat L, Henriet J, Lapayre JC. Conflict management in the fusion of complementary segmentations of deformed kidneys and nephroblastoma. Med Image Anal 2020;60:101629. [Crossref] [PubMed]
- Zhang J, Hou T, Qi X, et al. SOX21-AS1 is associated with clinical stage and regulates cell proliferation in nephroblastoma. Biosci Rep 2019;39:BSR20190602. [Crossref] [PubMed]
- Fu W, Zhu J, Xiong SW, et al. BARD1 Gene Polymorphisms Confer Nephroblastoma Susceptibility. EBioMedicine 2017;16:101-5. [Crossref] [PubMed]
- Maslak PG, Dao T, Bernal Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv 2018;2:224-34. [Crossref] [PubMed]
- Chuk MK, Widemann BC, Minard CG, et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children's Oncology Group. Pediatr Blood Cancer 2018;65:e27077. [Crossref] [PubMed]
- Rivera B, Nadaf J, Fahiminiya S, et al. DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis. J Clin Invest 2020;130:1479-90. [Crossref] [PubMed]
- Chagaluka G, Paintsil V, Renner L, et al. Improvement of overall survival in the Collaborative Wilms Tumour Africa Project. Pediatr Blood Cancer 2020;67:e28383. [Crossref] [PubMed]
- Tang C, Zhou D, Tan R, et al. Auxiliary genetic analysis in a Chinese adolescent NPH family by single nucleotide polymorphism screening. Mol Med Rep 2020;21:1115-24. [Crossref] [PubMed]
- Yin Y, Cao H, Zou H. Influence of psychological nursing intervention in the recovery of children with Wilms' tumor. Minerva Pediatr 2019;71:545-7. [Crossref] [PubMed]
- Ehrlich PF, Chi YY, Chintagumpala MM, et al. Results of Treatment for Patients With Multicentric or Bilaterally Predisposed Unilateral Wilms Tumor (AREN0534): A report from the Children's Oncology Group. Cancer 2020;126:3516-25. [Crossref] [PubMed]
- Lin A, Fu W, Wang W, et al. Association between PHOX2B gene rs28647582 T>C polymorphism and Wilms tumor susceptibility. Biosci Rep 2019;39:BSR20192529. [Crossref] [PubMed]
- Rautenberg C, Germing U, Pechtel S, et al. Prognostic impact of peripheral blood WT1-mRNA expression in patients with MDS. Blood Cancer J 2019;9:86. [Crossref] [PubMed]
- Gavens E, Arul GS, Pachl M. A single centre matched pair series comparing minimally invasive and open surgery for the resection of pediatric renal tumours. Surg Oncol 2020;35:498-503. [Crossref] [PubMed]
- Koshinaga T, Takimoto T, Okita H, et al. Blastemal predominant type Wilms tumor in Japan: Japan Children's Cancer Group. Pediatr Int 2019;61:351-7. [Crossref] [PubMed]
- Irtan S, Messahel B, Moroz V, et al. Outcomes of non-anaplastic stage III and 'inoperable' Wilms tumour treated in the UKW3 trial. Radiother Oncol 2019;131:1-7. [Crossref] [PubMed]
- Richards MK, Goldin AB, Ehrlich PF, et al. Partial Nephrectomy for Nephroblastoma: A National Cancer Data Base Review. Am Surg 2018;84:338-43. [Crossref] [PubMed]
- Liu XH, Zhang CM, Pan PQ, et al. Long noncoding RNA KCNQ1OT1 contributes to the development of nephroblastoma via modulating miR-21/PTEN axis. J Biol Regul Homeost Agents 2020;34:1901-8. [PubMed]
- Doganis D, Panagopoulou P, Tragiannidis A, et al. Childhood nephroblastoma in Southern and Eastern Europe and the US: Incidence variations and temporal trends by human development index. Cancer Epidemiol 2018;54:75-81. [Crossref] [PubMed]
- Wang HF, Wang WH, Zhuang HW, et al. MiR-429 regulates the proliferation and apoptosis of nephroblastoma cells through targeting c-myc. Eur Rev Med Pharmacol Sci 2018;22:5172-9. [PubMed]
- Rahmati-Holasoo H, Soltani M, Masoudifard M, et al. Nephroblastoma in bester sturgeon, a cultured hybrid of Huso huso × Acipenser ruthenus: Diagnostic imaging, clinical and histopathological study. J Fish Dis 2018;41:1093-101. [Crossref] [PubMed]
- Wiles AB, Powers CN, Smith SC. Reply to GATA3 differential expression in neuroblastoma and nephroblastoma. Cancer Cytopathol 2018;126:216-7. [Crossref] [PubMed]
- Hisada Y, Mackman N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin Thromb Hemost 2019;45:385-95. [Crossref] [PubMed]
- Zhang PC, Liu X, Li MM, et al. AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1α/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo. Biochem Pharmacol. 2020;172:113771. [Crossref] [PubMed]
- Lin YW, Huang ST, Wu JC, et al. Novel HDGF/HIF-1α/VEGF axis in oral cancer impacts disease prognosis. BMC Cancer 2019;19:1083. [Crossref] [PubMed]
- Itatani Y, Yamamoto T, Zhong C, et al. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci U S A 2020;117:21598-608. [Crossref] [PubMed]
- Hosaka K, Yang Y, Seki T, et al. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat Commun 2020;11:3704. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)





