
Safety of corticosteroids in the treatment of acute respiratory disease in children: a systematic review and meta-analysis
Introduction
Respiratory diseases in children include acute and chronic inflammation of the upper and lower respiratory tract, of which acute respiratory tract infection is the most common, and accounts for more than 60% of pediatric outpatient cases (1). Respiratory tract infections in children can be caused by viruses (mainly rhinovirus, respiratory syncytial virus, and influenza virus) or bacteria (2,3). Respiratory tract infections in children caused by viruses or bacteria can be treated with antibiotics (often penicillin and macrolides), and bronchodilators (e.g., salbutamol), which relax bronchial smooth muscles, dilate bronchi and relieve airflow limitation (4). Corticosteroids can reduce airway hyperresponsiveness, eliminate airway inflammation, repair damaged epithelium and promote the regeneration of airway cilia. They are often used in the treatment of acute asthma or asthma caused by respiratory tract infection. However, whether oral therapy or inhalation therapy, long-term treatment may affect the function of hypothalamic pituitary adrenal axis in asthmatic children, affecting children’s growth and development (5). A research by Toogood (6) showed that for adult respiratory tract patients, the application of high-dose corticosteroids could more effectively reduce airway response than low-dose corticosteroids, but the inhibitory effect of adrenocortical function was more obvious. Therefore, when applied in children, the dosage of corticosteroids should be strictly limited. The results of a meta-analysis by Garrison et al. (7) showed that oral corticosteroids had a significant positive effect in children aged <1 year with respiratory disease by shortening the hospitalization time and symptom duration of children, and there were no obvious adverse reactions in short-term treatment. However, another randomized control trial (RCT) (8) found that oral corticosteroids had no significant effect in children aged 1–5 years. According to the meta-analysis carried out by Fernandes et al. (9), corticosteroids are effective in children with a few adverse event cases, but the administration methods limited to inhalation in this study, lacking of literatures on oral methods, and the quality and accuracy of the included studies are low. We conducted a meta-analysis of controlled clinical studies with better quality to determine the true effect and safety of corticosteroid drugs in the treatment of clinical pediatric respiratory diseases in children. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-577/rc).
Methods
Inclusion of studies
To be eligible for inclusion in the meta-analysis studies had to meet the following inclusion criteria: (I) be an RCT published after January 2000 in the language English (non-RCT studies of individual cases, guidelines, systematic analyses, and case-control studies were excluded); (II) comprise patients aged <18 years old, who had been diagnosed with acute respiratory tract infection, with or without wheezing symptoms, with runny nose and body temperature symptoms (>37.5 °C) (children with chronic asthma, tuberculosis, congenital heart disease, corticosteroid contraindications, and those who had receive any anti-inflammatory drug treatment before the study were excluded); (III) use one of the following intervention methods: randomization, double-blinded, placebo-controlled studies were preferred. Studties should have a sample size of >10 patients for both intervention and control group, in which the patients received the same basic treatment (i.e., disease assessment, data collection, antibiotic therapy, and bronchodilator therapy, such as salbutamol). For those in the experimental group patients received corticosteroid therapy (i.e., prednisone, methylprednisolone, hydrocortisone, dexamethasone, or budesonide) and those in the control group patients received placebos; 4) detail the outcome indicators, the statistical methods, and the data in the article, or have linkage for the data.
Article search strategy
The following databases were searched: Embase (January 2000 to August 2021), PubMed (January 2000 to August 2021), Web of Science (January 2000 to August 2021), Ovid (January 2000 to August 2021), and ClinicalTrials.org. The keyword rapid search method was used, and the input keywords were “corticosteroids” and “acute respiratory diseases.”
Selection of articles
Two researchers independently screened the articles based on the above-mentioned criteria, and if any issues arose during this process, a 3rd person was consulted for any contradiction.
Data extraction
Excel was used to extract data. The following data were extracted: (I) basic data about the article: publication time, author, and region; (II) characteristics of the study subjects: patient age, sex ratio, ethnicity, family history of asthma, history of hormone use, and initial Preschool Respiratory Assessment Measure (PRAM) score; (III) article intervention methods: the intervention methods of the 2 groups, and the observation time; and (IV) outcome data. In the process of data extraction, if the data were provided in the article, the data were accessed via the address specified in the article. If the data could not be obtained from the address, the original author of the article was contacted to obtain the data. If the data still could not be obtained, the article was excluded.
Outcome indicators
The short-term efficacy indicators were as follows: (I) hospitalization rate; (II) proportion of patients who were not cured after treatment for 3 days; and (III) incidence rate of adverse reactions, including vomiting, maculopapular rash, diarrhea and restlessness. The long-term efficacy indicator was the readmission rate.
The following indicators were not subject to a statistical analysis: comparison of PRAM scores at 4, 8, and 12 h after treatment; the dosage of salbutamol during treatment, the proportion of cure after 7 days of treatment, the average time required for the symptoms to completely disappear, the serum hypersensitivity-C reactive protein (hsCRP) and interleukin (IL)-8 levels, and the number of days the patient presented with a cough. We did not perform a statistical analysis on these indicators as: (I) the number of articles reporting on some of the indicators was too small (e.g., only 1–2 articles reported on the dosage of salbutamol); (II) the reports on the indicators in different studies were not uniform (e.g., for salbutamol, some studies counted the dosage for 2 days, and some counted the dosage throughout the treatment).
Statistical methods
The I2 test analysis and Q test were used to examine the heterogeneity between the different studies. A I2 value <50% or a P value ≥0.1 indicated that there was no statistically significant heterogeneity (or acceptable) heterogeneity between the articles. The mean difference (MD) was used to report the effect size for the continuous variables, and the odds ratio (OR) was used to report the effect size for binary variables. A 95% confidence interval (CI) was used. A P value <0.05 was considered statistically significant. The results are displayed in forest plots. For each outcome indicator, all of the articles included in the meta-analysis that reported on this indicator were combined for the statistical analysis. If there was no statistically significant heterogeneity, a fixed-effects model was used. If there was heterogeneity, a random-effects model was used. Revman 5.4 software provided by Cochrane was used as the analysis tool in this study to present the results. The publication bias is shown in the funnel plot.
Risk of bias, heterogeneity survey, and sensitivity analysis
The risk of bias assessment tool provided in Revman 5.4 was used for the analysis. The risk assessment of each article was performed in relation to 6 factors: randomization, allocation concealment, quality of blinding, outcome assessment, incomplete data, selective reporting, and other bias. If there was heterogeneity in the statistical process, the source of heterogeneity was examined by a subgroup analysis. If the source could not be confirmed, a general descriptive analysis was conducted. The sensitivity analysis was performed by comparing the results of the fixed-effects model with those of the random-effects model.
Results
Article screening results
Following the database search, 544 articles were initially retrieved, and 8 articles with 2,327 patients were ultimately included in the meta-analysis. Articles were excluded if the pulmonary infection was caused by other primary diseases (10), the sample size was too small (11), or the outcome indicators were not examined (12). Figure 1 shows the selection flow chart.
Basic characteristics of articles
The basic characteristics of the articles are set out in Table 1. The subjects of the studies (13-20) were all children. The participants in a number of studies (13,14,16-18) were preschool aged children, while other articles (15) only specified that the children were aged <12 years old, and 2 articles (19,20) indicated that the children were aged <1 year old. The subjects of one study (15) were patients with community-acquired pneumonia, the subjects of two studies (19,20) had acute respiratory syncytial virus bronchiolitis, and the subjects of the other studies were children with viral upper respiratory tract infection and asthma. Only two studies (16,17) reported on the PRAM scores of the children before and after treatment. The shortest follow-up time was 7 days, and the longest was 3 months.
Table 1
| Author | Year of publication | Mean age (years) | Disease type | Population (E/C) | Intervention group | Control group | Follow-up time | Outcome Indicators |
|---|---|---|---|---|---|---|---|---|
| Foster et al. (13) | 2018 | 2–6 | Asthma due to viral upper respiratory tract infection | 305/300 | Oral prednisolone (1 mg/kg) once a day for 3 days | Placebo | 3 months | Length of hospital stay; proportion of patients who were not cured after 3 days; readmission rate; adverse reactions |
| Csonka et al. (14) | 2003 | 0.5–3 | Viral respiratory infection | 113/117 | Oral prednisolone 2 mg/kg/day for 3 days | Placebo | 21 days | Hospitalization rate; length of hospital stay; proportion of patients who were not cured in 3 days; re-hospitalization rate; adverse reactions |
| Nagy et al. (15) | 2013 | 1–12 | Community-acquired pneumonia | 29/30 | Imipenem + 5-day adjuvant methylprednisolone therapy | Imipenem + 5% dextrose | 2 months | Time of fever; hsCRP level; length of hospital stay; adverse event rate |
| Wallace et al. (16) | 2021 | 2–5 | Asthma due to respiratory disease | 238/239 | 2 mg/kg (maximum 40 mg) oral prednisolone once daily for 3 days | Placebo | 1 month | Length of stay; hospitalization rate; length of stay; PRAM score; adverse reaction rate |
| Panickar et al. (17) | 2009 | 0.5–5 | Asthma due to viral upper respiratory tract infection | 343/344 | 5-day course of oral prednisolone (10 mg once a day for children under 2 years old, 20 mg once a day for older children) | Placebo | 7 days | Hospital stay; dose of salbutamol used; PRAM score; proportion of patients who were not cured in 3 days; complete recovery time; re-hospitalization rate |
| Jartti et al. (18) | 2007 | 0.5–5 | Asthma due to viral upper respiratory tract infection | 23/36 | Oral prednisolone (first dose 2 mg/kg, then 2 mg/kg/d, divided into 3 doses for 3 days; a maximum dose of 60 mg/d | Placebo | 2 months | Length of stay; re-hospitalization rate |
| Bentur et al. (19) | 2005 | 0.5–1 | Respiratory syncytial virus bronchiolitis | 29/32 | 0.25 mg inhaled Dexamethasone + 1 mL epinephrine | 0.5 mL 0.9% + saline 1 mL epinephrine | 1 month | Hospital stay |
| Cade et al. (20) | 2000 | 0.5–1 | Acute respiratory syncytial virus bronchiolitis | 82/79 | 1 mg of nebulised budesonide (Bud) twice daily from admission until 2 weeks after discharge | Placebo | 1 month | Length of stay; readmission rate |
E indicates the intervention group and C indicates the control group.
Risk assessment of bias of included articles
Revman 5.4 was used to conduct the risk assessment of the included articles. All of the articles included detailed descriptions of the random grouping method and drop-out cases. No selective reporting bias risk or other risk was found. One article (15) did not adopt the blind method, which increased the risk of implementation bias. One article (18) did not specify the use of allocation concealment or the blind method, which may indicate selective risk (see Figures 2,3).
Meta-analysis results
Length of stay
All of the included articles (13-20) reported on the length of the hospital stay (pediatric emergency room or general ward). As there was great heterogeneity between the articles (I2=95%, P<0.00001), the random-effects model was used. The length of hospital stay of the experimental group treated with corticosteroids was shorter than that of the control group (MD =–2.03, 95% CI: –2.91, –1.14; P<0.00001; see Figure 4).

Based on the disease type of the children, the patients were further divided into 3 subgroups (i.e., the viral wheeze group, the community-acquired pneumonia group, and the viral respiratory bronchiolitis group). The results showed the use of corticosteroids shortened the length of hospital stay in the viral wheeze group (MD =–2.26, 95% CI: –3.31, –1.22; P<0.0001), the community-acquired pneumonia group (MD =–5.00, 95% CI: –7.03, –2.97; P<0.0001), and the viral respiratory bronchiolitis group (MD =–0.56, 95% CI: –0.96, –0.15; P=0.007).
Proportion of patients who did not recover after 3 days of treatment
Three articles (comprising a total of 1,519 patients) (13,14,17) reported on the number and proportion of patients who did not recover after 3 days of treatment. The number of patients who did not recover after 3 days of treatment in the experimental group and the control group was 759 and 760, respectively. As there was no statistical heterogeneity between the articles (I2=42%, P=0.18), the fixed-effects model was used. Corticosteroid drug therapy reduced the number and proportion of uncured patients after 3 days of treatment (OR =0.55, 95% CI: 0.42, 0.72; P<0.00001; see Figure 5).
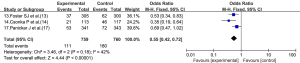
Adverse reactions
Four articles (13-16) reported on the adverse reactions of patients treated with corticosteroid drugs. As there was no statistical heterogeneity between the articles (I2=0%, P=0.91), the fixed-effects model was used. The adverse reactions of patients in the experimental group were not significantly different from those of patients in the control group (OR =0.57, 95% CI: 0.31, 1.07; P=0.08; see Figure 6).
Long-term efficacy: re-hospitalization rate
Five articles (13,14,17,18,20) reported on the re-hospitalization rate of children after treatment. As there was no statistical heterogeneity between the articles (I2=0%, P=0.77), the fixed-effects model was used. The readmission rate of the experimental group did not differ significantly to that of the control group (OR =0.94, 95% CI: 0.66, 1.34; P=0.75; see Figure 7).
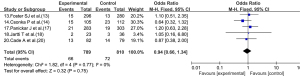
Heterogeneity investigation and sensitivity analysis
In the indicator analysis of the length of hospital stay, there was statistically significant heterogeneity among the articles. Thus, the articles were analyzed according to the type of disease. However, there was still heterogeneity among the articles in each subgroup. The source of heterogeneity may be related to multiple confounding factors, such as the age, race, and type and dose of the corticosteroid drugs. In the analysis of the re-hospitalization rate, when the random-effects model was used, the results did not differ significantly to those of the fixed-effects model; thus, the results can be considered stable (sensitivity analysis).
Analysis of publication bias
In the analysis of the re-hospitalization rate, the funnel plot showed that both sides were evenly distributed, which suggests that any publication bias was only small (see Figure 8).
Discussion
Eight articles, involving 4 kinds of corticosteroids (i.e., prednisolone, methylprednisolone, dexamethasone, and budesonide) and 3 kinds of pediatric respiratory diseases (viral infection with asthma, community-acquired pneumonia, and viral respiratory bronchiolitis), were included in this study. Except for 1 article (15), the study subjects in the other articles were preschool aged children. Some studies (16,17) have found that the effect of oral corticosteroids in treating pediatric respiratory diseases was not superior to placebos, but the results of the final comprehensive analysis showed that the experimental group treated with corticosteroids had shorter hospital stays and higher cure rates after 3 days of treatment than the placebo group, which suggests that treatment with corticosteroids significantly shortened the treatment time. In the long term, corticosteroid use did not further improve treatment outcomes (the readmission rate of the experimental group was similar to that of the placebo group), but the short-term outcomes could still benefit children. Corticosteroid drugs have a powerful effect, allowing children to achieve an immunoprotective state in a short period, thereby improving symptoms (21). Some studies (22) have also shown that treatment with corticosteroid drugs can accelerate the digestion of the virus, improve the immune capacity of children, and reduce serum inflammation.
However, the heterogeneity analysis of the articles in this study showed that there were great differences in the types, doses, and indications of corticosteroid drugs, and the age of the children and the types of diseases were also important factors that cause heterogeneity. Thus, the safety of corticosteroid drugs requires further study. In this meta-analysis, 4 articles (13-16) reported on the children’s adverse reactions to corticosteroid drugs. However, the results showed that oral corticosteroid drugs did not increase the adverse reactions, but also did not increase the long-term readmission rate of children. Strouse (23) concluded that while dexamethasone has been shown to be effective at reducing the duration of respiratory disease due to sickle cell anemia, the great risk of readmission for pain should limit its use; however, none of the studies in this meta-analysis reported that the use of corticosteroids caused an increase in pain.
Only 8 articles were included in this meta-analysis. The article quality was good; however, some of the articles did not use the blind method, which may have caused an implementation bias. The funnel plots revealed no publication bias, but the number of included articles was small, as was the sample size. Due to the heterogeneity of the articles, relevant higher quality large-sample size RCT studies need to be conducted to gather further evidence.
Conclusions
Eight articles were included in this meta-analysis. The results showed that the application of corticosteroids in the treatment of children with respiratory diseases significantly shortened hospitalization stay and increased the cure rate of patients without increasing adverse reactions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-577/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-577/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nascimento AP, Santos JM, Mill JG, et al. Association between the incidence of acute respiratory diseases in children and ambient concentrations of SO2, PM10 and chemical elements in fine particles. Environ Res 2020;188:109619. [Crossref] [PubMed]
- Sharma BS, Shekhawat DS, Sharma P, et al. Acute Respiratory Distress in Children: Croup and Acute Asthma. Indian J Pediatr 2015;82:629-36. [Crossref] [PubMed]
- Richardson AE, Warrier K, Vyas H. Respiratory complications of the rheumatological diseases in childhood. Arch Dis Child 2016;101:752-8. [Crossref] [PubMed]
- Kavitha TK, Angurana SK. Long-Term Respiratory Morbidity Among Children With Acute Respiratory Failure: More to Discover. Crit Care Med 2020;48:e1368-e1369. [Crossref] [PubMed]
- Mammen MJ, Aryal K, Alhazzani W, et al. Corticosteroids for patients with acute respiratory distress syndrome: a systematic review and meta-analysis of randomized trials. Pol Arch Intern Med 2020;130:276-86. [Crossref] [PubMed]
- Toogood JH, Lefcoe NM, Haines DS, et al. A graded dose assessment of the efficacy of beclomethasone dipropionate aerosol for severe chronic asthma. J Allergy Clin Immunol. 1977;59:298-308. [Crossref] [PubMed]
- Garrison MM, Christakis DA, Harvey E, et al. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics 2000;105:E44. [Crossref] [PubMed]
- Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for children aged 1-5 years: randomised controlled trial. Lancet 2003;362:1433-8. [Crossref] [PubMed]
- Fernandes RM, Wingert A, Vandermeer B, et al. Safety of corticosteroids in young children with acute respiratory conditions: a systematic review and meta-analysis. BMJ Open. 2019;9:e028511. [Crossref] [PubMed]
- Webb NJ, Frew E, Brettell EA, et al. Short daily course prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid-sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014;15:147. [Crossref] [PubMed]
- Bellodi S, Tosca MA, Pulvirenti G, et al. Activity of budesonide on nasal neutrophilic inflammation and obstruction in children with recurrent upper airway infections. A preliminary investigation. Int J Pediatr Otorhinolaryngol 2006;70:445-52. [Crossref] [PubMed]
- van Woensel JB, Vyas HSTAR Trial Group. Dexamethasone in children mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus: a randomized controlled trial. Crit Care Med 2011;39:1779-83. [Crossref] [PubMed]
- Foster SJ, Cooper MN, Oosterhof S, et al. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial (published correction appears in Lancet Respir Med. 2018 Jan 31;). Lancet Respir Med 2018;6:97-106. [Crossref] [PubMed]
- Csonka P, Kaila M, Laippala P, et al. Oral prednisolone in the acute management of children age 6 to 35 months with viral respiratory infection-induced lower airway disease: a randomized, placebo-controlled trial. J Pediatr 2003;143:725-30. [Crossref] [PubMed]
- Nagy B, Gaspar I, Papp A, et al. Efficacy of methylprednisolone in children with severe community acquired pneumonia. Pediatr Pulmonol 2013;48:168-75. [Crossref] [PubMed]
- Wallace A, Sinclair O, Shepherd M, et al. Impact of oral corticosteroids on respiratory outcomes in acute preschool wheeze: a randomised clinical trial (published correction appears in Arch Dis Child. 2021 Mar 30;). Arch Dis Child 2021;106:339-44. [Crossref] [PubMed]
- Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med 2009;360:329-38. [Crossref] [PubMed]
- Jartti T, Lehtinen P, Vanto T, et al. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr Allergy Immunol 2007;18:326-34. [Crossref] [PubMed]
- Bentur L, Shoseyov D, Feigenbaum D, et al. Dexamethasone inhalations in RSV bronchiolitis: a double-blind, placebo-controlled study. Acta Paediatr 2005;94:866-71. [Crossref] [PubMed]
- Cade A, Brownlee KG, Conway SP, et al. Randomised controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child 2000;82:126-130. [Crossref] [PubMed]
- Silverman M, Sheffer AL, Díaz PV, et al. Safety and tolerability of inhaled budesonide in children in the Steroid Treatment As Regular Therapy in early asthma (START) trial. Pediatr Allergy Immunol 2006;17:14-20. [Crossref] [PubMed]
- van Woensel JB, Lutter R, Biezeveld M, et al. Effect of dexamethasone on viral load and interleukin-8 tracheal concentration in children with respiratory syncytial virus infection. Pediatr Infect Dis J 2003;22:721-6. [Crossref] [PubMed]
- Strouse JJ, Takemoto CM, Keefer JR, et al. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer 2008;50:1006-12. [Crossref] [PubMed]







