Real-time shear wave elastography evaluation of the correlation between brain tissue stiffness and body mass index in premature neonates
Introduction
Neonates are prone to intracranial hemorrhage due to their imperfect cranial brain structure, which can lead to a series of cognitive and neurological dysfunctions. Ultrasonography is non-invasive, convenient, and efficient, and is currently the main method for the clinical screening and follow-up of neonatal craniosynostosis (1). Shear wave elastography (SWE) is an ultrasonic imaging method based on detecting the propagation of shear wave in tissue (2). It can be used to quantitatively measure tissue stiffness in a region of interest (3). SWE has been widely used as an aid in ultrasound diagnoses of diseases of abdominal organs, superficial organs, and musculoskeletal nerves. Tzschätzsch et al. have published a paper demonstrated that SWE can detect acute cerebral stiffness changes induced by intracranial pressure variations in a total of 26 healthy adults (4).
Elastic modulus (Emean) which measures the resistance of the material to elastic—or “springy”—deformation (5). The elasticity of brain tissue was found to be significantly lower in the preterm group compared with the term group (6). Additionally, brain tissue elasticity has been thought to be a factor affecting brain re-expansion after the evacuation of intracranial hemorrhage (7). Furthermore, premature neonates tended to have lower elasticity of brain tissue and lower body mass index (BMI) compared with the full-term infants.
However, little research has been conducted on using SWE in the diagnosis of neonatal craniocerebral disorders. In this study, we compared the Emean of the lateral paraventricular brain white matter, thalamus, and choroid in premature and full-term neonates, and also analyzed the related factors affecting brain tissue stiffness in neonates and its association with BMI. We present the following article in accordance with the MDAR reporting checklist (available at https://dx.doi.org/10.21037/tp-21-513).
Methods
General information
The data of 159 neonates admitted to our hospital from December 2019 to February 2021 were collected. Of the 159 neonates, 76 were premature with a mean gestational age of 32.93±1.85 weeks and a body mass index (BMI) of 10.70±1.01, and 83 were full-term with a mean gestational age of 39.22±1.02 weeks and a BMI of 12.94±0.83. The neonates’ age ranged from 0 to 28 days. Premature neonates, full-term neonates, and neonates with neonatal pneumonia were included in this study. Neonates with hyperbilirubinemia, intracranial hemorrhage, ischemic-hypoxic encephalopathy, hydrocephalus, amniotic fluid contamination, or intracranial tumors were excluded from the study. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Lanzhou University Second Hospital (No.: 2021A-598) and all the guardians of the neonates gave informed consent for the neonates to participate in this study.
Instruments and methods
A color Doppler ultrasound diagnostic instrument from Supersonic Imagine Aixplorer, France with a probe frequency of SC6-1 MHz was used. The examination room was warm and comfortable, and the neonates were examined in the supine position in a quiet state. The cranial structures were observed via the fontanelle and lateral fontanelle in 2-dimensional (2D) grayscale mode, and cerebral blood flow parameters were detected in color Doppler mode. Neonates who met the above-mentioned criteria were included in the study (3).
Switching to the SWE mode, the Emeans of the affected neonate’s left/right paraventricular white matter, thalamus, and choroid were measured via a sagittal sweep of the fontanelle, and the Emeans were averaged over 3 measurements. Young’s modulus value (E=3ρc2, where E represents the tissue elastic modulus, ρ represents the tissue density, and c represents the shear wave propagation velocity) were automatically calculated by the machine (8). All procedures were performed by the same physician. A coupling agent was applied thickly during the examination; only minimal pressure was placed on the affected fontanelle. The area of interest for the Emean measurement in the paraventricular cerebral white matter was placed in the area of the cerebral white matter at the peripheral edge of the corpus callosum. The area of interest for the Emean measurement in the thalamus was placed in the middle of the thalamus. The area of interest for the Emean measurement in the choroid was placed at the head of the choroid near the caudate sulcus of the thalamus. A 3 cm × 4 cm sampling frame size was chosen to display the 3 regions of interest simultaneously and reduce the sampling time.
Statistical methods
SPSS 24.0 statistical software was used to process the data. Measures conforming to a normal distribution are expressed as mean ± standard deviation (). The t-test was used for comparisons between groups. Correlations between neonatal BMI and the Emeans of selected regions of the bilateral lateral paraventricular brain white matter, thalamus, and choroid were analyzed using Pearson’s correlation coefficient. A P value <0.05 was considered statistically significant.
Results
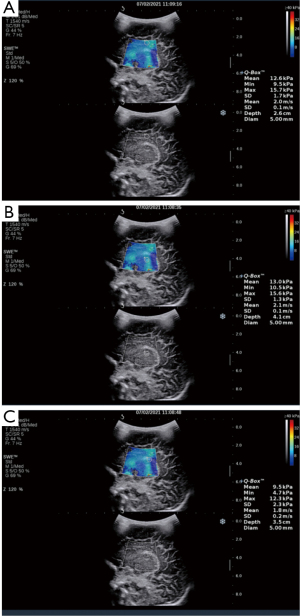
- The Emean measurements of the SWE sonogram for the regions of interest in the neonatal cranial lateral ventricular parietal cerebral white matter, thalamus, and choroid are shown in Figure 1.
- The Emeans of the lateral paraventricular white matter, thalamus, and choroid were lower in the premature neonate group than the full-term neonates control group (P<0.001). The Emean of the neonatal brain tissue thalamus was > lateral paraventricular brain white matter, which was > choroid (see Table 1).
Table 1
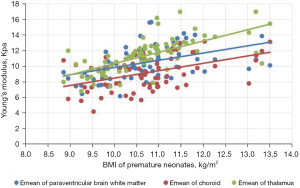
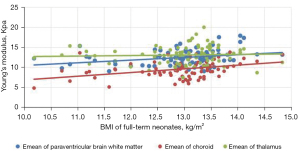
Comparison of Emean of the neonatal brain between premature neonates and full-term neonatesVariables Premature neonates (n=76) Full-term neonates (n=83) Left (kPa) Right (kPa) Mean (kPa) Left (kPa) Right (kPa) Mean (kPa) Lateral paraventricular white matter 10.39±2.10a 10.23±2.08a 10.50±2.04a 12.32±2.14 12.65±2.09 12.49±1.91 Thalamus 11.14±2.33a 11.25±2.07a 11.38±2.16a 13.31±2.49 12.92±2.40 13.11±2.22 Choroid 9.18±2.19a 9.15±2.17a 9.16±2.07a 9.65±2.24 9.62±2.20 9.64±2.08 a, P<0.001 premature neonate group vs. full-term neonate group. Emean, elasticity modulus. - The Pearson’s correlation coefficient analysis showed that the neonatal lateral paraventricular brain white matter, thalamus, and choroid brain tissue stiffness values were significantly and positively correlated with BMI (P<0.05). The correlation coefficients between the lateral paraventricular brain white matter, thalamus, and choroid and BMI were r=0.457, r=0.681, and r=0.462, respectively, for the premature neonate group and r=0.476, r=0.327, and r=368, respectively, for the full-term neonate control group (see Figures 2,3
).
Discussion
The imperfect development of cranial structures in premature neonates increases the risk of cognitive impairment, neuromotor deficits, and neurodevelopmental abnormalities (9,10). In addition to being convenient and inexpensive, with the improvement of probe resolution, ultrasonography has high diagnostic value in the diagnosis of neonatal cranial diseases, and can be combined with color Doppler imaging to assess the functional status of intracranial cerebral blood flow (3,11). Ultrasonography is currently the preferred method for the routine screening of neonatal early cranial diseases. However, the clinical ultrasound examination techniques currently and commonly used are susceptible to the subjective judgment of different operators. Thus, the provision of more quantitative analysis data would improve the sensitivity and specificity of ultrasonography in neonatal cranial applications.
The SWE is an ultrasound imaging technique that has emerged in recent years. This technique acts on the acoustic radiation force emitted by the ultrasound probe in the tissue region of interest, and then measures the shear wave propagation velocity in the tissue and automatically calculates Young’s modulus value (E=3ρc2, where E represents the tissue elastic modulus, ρ represents the tissue density, and c represents the shear wave propagation velocity) (8) to quantitatively assess tissue stiffness. Currently, SWE is widely used clinically; however, its application to neonatal cranial brain research is still in the preliminary stage (6,12,13).
Neonatal central nervous system development is highly sensitive to external sources, and disturbances of the developmental process can have important effects on brain tissue structure and function (14,15). Research has been conducted on the safety of 2D-SWE in craniocerebral examinations. For example, Li et al. (12) used shear wave imaging to irradiate the cranial brains of different groups of newborn C57BL/6 rats, and found that 30 min of irradiation did not cause significant changes to the brain tissue structure of these newborn rats. However, the newborn rats showed decreased brain protein kinase Ca (p-PKCa) protein expression levels after >10 min of irradiation, and the brain phosphatidylinositol-3-kinase mammalian target of rapamycin signaling pathway was disturbed after >30 min. The results of another study (16) showed that there was no significant difference in learning and memory between experimental and control groups when the experimental rats were fed to 3 months of age, and there was no significant difference in the expression of proteins related to signaling pathways closely related to neurological development in the hippocampus and cortex of the 2 groups of rats. In the present study, the difference in the Emean data of brain tissue in the same region was small and the image display quality was high when the SWE mode of the C5-1 probe was used to measure brain tissue. Further, to reduce the total time of the ultrasound examination and thus minimize the possible biological effects that may occur during the cranial ultrasonography of neonates, the present study showed that elasticity information of brain tissue in the lateral paraventricular brain white matter, thalamus, and choroid could be simultaneously acquired when the SWE sampling frame size was 3 cm × 4 cm.
In this study, the Emean of the thalamus was > the Emean of the lateral paraventricular brain white matter, which was > the choroid in the brain tissue of both premature and full-term neonates. Few studies (6,13,16) showed that SWE can effectively measure brain tissue stiffness values in all the brain regions of neonates, and Albayrak et al. (6) proposed that the cut-off points of Young’s modulus values of lateral paraventricular brain white matter and thalamus in normal neonates were 6.59 and 8.28 kPa, respectively. Compare with the present study, both the white matter and thalamus Young’s modulus values were smaller. During the procedure in the present study, there were some differences in the measured values of brain tissue stiffness in the same region when the SWE probe frequencies were performed. Notably, the C5-1 probe in a SWE mode was used to collect the intracranial brain tissue measurements for Young’s modulus values, which has better reproducibility than the higher frequency probe used to collect data in the present study. Tavare et al. (13) showed that Young’s modulus values of the thalamus, cerebellum, and lateral paraventricular brain white matter in children with ischemic-hypoxic encephalopathy differed from those of normal full-term neonates, and suggested that SWE could provide a basis for the clinical diagnosis of brain injury. However, very few studies have examined the correlation between neonatal BMI and brain tissue elasticity values.
The gray matter of the brain is the dense site of neuronal cell bodies, and the white matter of the brain is the site of nerve fiber aggregation. When the thalamic elastic stiffness values are higher than the white matter stiffness values in the neonatal period, the gray matter structure is denser than the white matter structure of the brain. The development of the central nervous system is an extremely complex and continuous process that involves a dynamic balance between cell proliferation, differentiation, migration, synaptogenesis, myelin formation, and apoptosis of brain neurons and glia. Examinations of correlations between brain tissue stiffness and the degree of cerebral vascular development and cerebral blood perfusion pressure have shown that the denser the neuronal cells, the higher the value of brain tissue stiffness (17). In this study, we analyzed correlations between BMI and brain tissue Emeans in premature and full-term neonates, and found that the higher the BMI, the higher the neonatal thalamus, lateral paraventricular cerebral white matter, and choroid plexus tissue stiffness. Pong et al. (18) used Magnetic resonance elastography (MRE) to examine the cranial brains of rats at 1–6 weeks, and the results suggested that the brain gray matter stiffness of young rats increases with age. Which provides support for the results of the present study.
There are some advantages and disadvantages of SWE in evaluation of brain tissue stiffness. Stiffness measurements do not take a matter of minutes by SWE, and intraoperative ultrasonography is a relatively safety approach. Additionally, SWE can provide dynamic real-time images while not impacted by brain shift (19,20). Furthermore, the major flaws of using SWE to evaluate brain tissue stiffness including, stiffness values that may not be acquired when depth and/or obstacle forbid shear wave propagation, different ultrasonography operators may result in distinct results due to their different skills (19,21).
This study had a number of limitations. First, the subjects in this study were relatively mobile, regular measurements of Young’s modulus values of the brain tissue in the same sample could not be collected. Second, the examination sites in this study were limited to the detection of the Emeans of the bilateral lateral paraventricular cerebral white matter, thalamus, and choroid. Future research should collect multi-site measurements and conduct a systematic analysis of large sample size data to provide a range of brain tissue elastic stiffness values at different stages of neonatal life.
In summary, the SWE technique can be used to transcranially quantify brain tissue stiffness in premature and full-term neonates. The sample size should be expanded to establish a database of normal brain tissue stiffness values for neonates to provide a reference basis for the clinical diagnosis of neonatal craniosynostosis.
Acknowledgments
Funding: The study was supported by the Natural Science Foundation of Gansu Province (No.: 20JR5RA346, project title: Application of shear wave elastography to neonatal ischemic-hypoxic brain injury).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://dx.doi.org/10.21037/tp-21-513
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tp-21-513
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tp-21-513). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Lanzhou University Second Hospital (No.: 2021A-598) and all the guardians of the neonates gave informed consent for the neonates to participate in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li A. Hemodynamic changes of cerebral artery in newborns with hypoxic-ischemic encephalopathy and it’s effect on the prognosis. Chinese Journal of Practical Nervous Diseases 2018;21:1088-92.
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:396-409. [Crossref] [PubMed]
- Tang Y, Cheng S, Tang X, et al. Quantification of skin lesions using high-frequency ultrasound and shear wave elastography in port-wine stain patients: a clinical study. Ann Transl Med 2019;7:803. [Crossref] [PubMed]
- Tzschätzsch H, Kreft B, Schrank F, et al. In vivo time-harmonic ultrasound elastography of the human brain detects acute cerebral stiffness changes induced by intracranial pressure variations. Sci Rep 2018;8:17888. [Crossref] [PubMed]
- Jones DRH, Ashby MF. Chapter 3 - Elastic Moduli, in Engineering Materials 1 (Fifth Edition). Butterworth-Heinemann 2019;31-47.
- Albayrak E, Kasap T. Evaluation of Neonatal Brain Parenchyma Using 2-Dimensional Shear Wave Elastography. J Ultrasound Med 2018;37:959-67. [Crossref] [PubMed]
- Fukuhara T, Gotoh M, Asari S, et al. The relationship between brain surface elastance and brain reexpansion after evacuation of chronic subdural hematoma. Surg Neurol 1996;45:570-4. [Crossref] [PubMed]
- deCampo D, Hwang M. Characterizing the Neonatal Brain With Ultrasound Elastography. Pediatr Neurol 2018;86:19-26. [Crossref] [PubMed]
- Su Y, Ma J, Du L, et al. Application of acoustic radiation force impulse imaging (ARFI) in quantitative evaluation of neonatal brain development. Clin Exp Obstet Gynecol 2015;42:797-800. [PubMed]
- Bussmann N, El-Khuffash A. Future perspectives on the use of deformation analysis to identify the underlying pathophysiological basis for cardiovascular compromise in neonates. Pediatr Res 2019;85:591-5. [Crossref] [PubMed]
- Sarvazyan AP, Rudenko OV, Swanson SD, et al. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol 1998;24:1419-35. [Crossref] [PubMed]
- Li C, Zhang C, Li J, et al. An Experimental Study of the Potential Biological Effects Associated with 2-D Shear Wave Elastography on the Neonatal Brain. Ultrasound Med Biol 2016;42:1551-9. [Crossref] [PubMed]
- Tavare AN, Alfuraih AM, Hensor EMA, et al. Shear-Wave Elastography of Benign versus Malignant Musculoskeletal Soft-Tissue Masses: Comparison with Conventional US and MRI. Radiology 2019;290:410-7. [Crossref] [PubMed]
- El-Ali AM, Subramanian S, Krofchik LM, et al. Feasibility and reproducibility of shear wave elastography in pediatric cranial ultrasound. Pediatr Radiol 2020;50:990-6. [Crossref] [PubMed]
- Zhang C, Li C, Xu Z, et al. The effect of surgical and psychological stress on learning and memory function in aged C57BL/6 mice. Neuroscience 2016;320:210-20. [Crossref] [PubMed]
- Kim HG, Park MS, Lee JD, et al. Ultrasound Elastography of the Neonatal Brain: Preliminary Study. J Ultrasound Med 2017;36:1313-9. [Crossref] [PubMed]
- Bailey C, Huisman TAGM, de Jong RM, et al. Contrast-Enhanced Ultrasound and Elastography Imaging of the Neonatal Brain: A Review. J Neuroimaging 2017;27:437-41. [Crossref] [PubMed]
- Pong AC, Jugé L, Cheng S, et al. Longitudinal measurements of postnatal rat brain mechanical properties in-vivo. J Biomech 2016;49:1751-6. [Crossref] [PubMed]
- Mathon B, Amelot A, Carpentier A, et al. Intraoperative real-time guidance using ShearWave Elastography for epilepsy surgery. Seizure 2019;71:24-7. [Crossref] [PubMed]
- Ohue S, Kumon Y, Nagato S, et al. Evaluation of intraoperative brain shift using an ultrasound-linked navigation system for brain tumor surgery. Neurol Med Chir (Tokyo) 2010;50:291-300. [Crossref] [PubMed]
- Selbekk T, Brekken R, Indergaard M, et al. Comparison of contrast in brightness mode and strain ultrasonography of glial brain tumours. BMC Med Imaging 2012;12:11. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)