
Congenital central hypoventilation syndrome in neonates: report of fourteen new cases and a review of the literature
Introduction
Congenital central hypoventilation syndrome (CCHS; OMIM #209880) is a rare disorder of the autonomic nervous system with an estimated incidence of about 1 in 148,000 to 200,000 live births (1,2). The disease is characterized by central hypoventilation due to abnormally reduced ventilatory responses to hypercapnia and hypoxia and associated manifestations of autonomic dysfunction such as Hirschsprung disease (HSCR) and neural crest tumours (neuroblastoma, ganglioneuroblastoma, and ganglioneuroma). HSCR and neural crest tumours occur in association with CCHS in 20% and 6% of cases, respectively (3,4). It classically manifests in newborn babies who present with central apneas, hypoxemia, and hypoventilation that are most severe during sleep resulting in the need for assisted ventilation. While a subset of patients remain asymptomatic until an older age with a diagnosis of later-onset CCHS (5,6).
Recent studies have identified an autosomal dominant inherited heterozygous paired-like homeobox 2B (PHOX2B) gene mutation in CCHS cases (7). The PHOX2B gene locates on chromosome 4p12, encoding a highly conserved homeobox transcription factor of 314 amino acids with two short and stable polyalanine repeats of 9 and 20 residues. Over 90% of patients with CCHS are heterozygous for polyalanine repeat expansion mutations (PARMs) in PHOX2B that can range from 24 to 33 alanines, and remaining 10% of patients have heterozygous non-PARMs (NPARMs) that include missense, nonsense and frameshift mutations in the polyalanine repeat region or elsewhere in the PHOX2B coding sequence (8).
Since PHOX2B gene was identified as the disease-defining gene in 2003, about 1,000 cases of PHOX2B mutation-confirmed CCHS have been documented worldwide (6). Clinical diagnosis of CCHS in the first days of life is sometimes challenging as a result of overlap of symptoms and signs with other conditions such as neuromuscular disorders. However, there are a limited number of studies detailing clinical and genetic findings in neonates. What’s more, only isolated cases were reported in mainland China and clinical features as well as mutation analysis of Chinese neonates with CCHS were rarely described (9).
In the present study, we provided a description of 14 neonatal cases of CCHS in China and we conducted a search of the literature to identify previously reported CCHS cases with neonatal onset. The aim of this study is to help delineate clinical and genetic features of neonates with CCHS, and provide data on the genotype-phenotype correlation. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-303).
Methods
Neonates with genetic confirmed CCHS evaluated at Children’s Hospital of Fudan University from January 2014 to December 2019 were included in this study. Inclusion criteria consisted of: (I) patients with typical clinical findings in keeping with the Statement of American Thoracic Society and identification of a pathogenic variant in PHOX2B gene (6); (II) patients with suggestive clinical findings and identification of a pathogenic variant in PHOX2B gene. Patients in whom PHOX2B sequencing was negative were excluded. Clinical data including sex, age, birth weight, delivery, perinatal history, family history, clinical manifestations, laboratory tests, ventilatory support (invasive vs. non-invasive; full time vs. sleep only) and outcome were collected. Children’s Hospital of Fudan University, is a national children’s medical center and a major tertiary referral center in east of China with more than 8,000 critically ill newborns treated per year.
This study was approved by the ethics committee of the Children’s Hospital of Fudan University [2015 (no. 169)] and adhered to the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from patients’ guardians.
Molecular analysis
Analysis of PHOX2B gene variants were performed through direct Sanger sequencing. When possible, parents were screened for the variants found in the proband.
DNA was extracted from peripheral blood lymphocytes using the QIAmp DNA Blood Mini Kit (Qiagen, Hilden, Germany). A Nano-Drop Spectrophotometer (ND-1000, Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the concentration and quality of the genomic DNA. The entire coding region and intron-exon boundaries of PHOX2B were sequenced after polymerase chain reaction amplification. The sequence reactions were analyzed on an ABI PRISM 3100 Genetic Analyzer. Sequence traces were analyzed with Mutation Surveyor. Novel variants of unknown significance were analyzed with in silico tools MutationTaster, SIFT and PolyPhen2.
Literature review
We reviewed articles in English containing neonatal-onset CCHS cases with identified PHOX2B mutations published in PubMed before January 2020. We recorded the following data: sex, gestational age, perinatal history, family history, clinical findings, genotypes, and other relevant specificities. Cases without genetic information, cases with combined chromosomal abnormalities and cases with insufficient clinical description were excluded. Based on respiratory manifestation, we defined mild-CCHS as no ventilatory support or requiring ventilatory support only during sleep, and severe-CCHS as requiring continuous ventilatory support in neonatal period. HSCR cases were classified as either long-segment HSCR (aganglionosis extended to above the splenic flexure or total colonic form) or short-segment HSCR (left-colonic or rectosigmoid forms) (10).
Statistics analysis
Descriptive statistics were used to analyze the demographic variables. Frequencies and percentages were presented for categorical variables. Mean and standard deviation were presented for continuous variables. Categorical variables were compared using either a Chi-squared test or a Fisher’s test when appropriate. A P value of <0.05 was considered to be statistically significant. The statistical analysis was performed with SPSS software, version 19.
Results
Clinical presentation of patients included in the Chinese cohort
Fourteen patients who met the criteria for diagnosis of CCHS were identified and had charts available for review. The mean gestational age was 38.7±2.2 weeks and the mean birth weight was 3,117.5±502.7 g. Eight (57.1%) of them were male. Patient 4 and Patient 12 were pair of male-female siblings. Three patients (21.4%) were born prematurely. Five patients (35.7%) had suspicious family history including early deaths, spontaneous miscarriages and affected sibling. The pregnancies of five cases (35.7%) were complicated by polyhydramnios. No facial anomalies were observed but one with floppy appearance. All the patients required invasive mechanical ventilation following intubation because of respiratory insufficiency including apnea, cyanosis, hypercapnia or repeated extubation failure in the neonatal intensive care unit (NICU). All the patients underwent chest radiograph and seven (50.0%) of them showed abnormal. Two patients (14.3%) had HSCR confirmed by biopsy and one had variant HSCR (with HSCR-like symptom but do not meet the histological diagnostic criteria for HSCR). Three patients (21.4%) had neurological complications of seizure. Cardiovascular defects were documented in 4 patients (28.6%) including atrial septal defect, patent ductus arteriosus and persistent left superior vena cava. No neural-crest tumor nor other autonomic dysfunction symptoms such as heart rate variability, body temperature regulation, or hypoglycemia were found in the cohort. No polysomnography was conducted because the patients were not stable enough to undergo such study. One patient died of sepsis in the hospital at the age of one month. Thirteen guardians (92.9%) elected withdrawal of treatment. Detailed clinical and laboratory tests findings of the 14 cases can be found in Table 1.
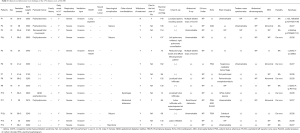
Full table
Genetic results in the Chinese cohort
Molecular analysis revealed that 10 cases had PARMs in PHOX2B (six with 26 PARM and four with 27 PARM) and the remaining cases had four different NPARMs including the previously reported c.202G>T (11), c.422G>A and c.722_759 del38 pathogenic variants (12,13) and the novel c.684dup variant which was predicted to cause frameshift and produce prolonged protein. Parents were analyzed in 6/14 cases and none PHOX2B pathogenic variants were identified.
Literature review of neonatal-onset CCHS cases
Our initial search identified 53 neonatal-onset CCHS cases (11,14-30) from 33 papers (31-45). After systematically reviewing, seven cases were excluded from our analyses because of lacking important information or co-occurrence of other genetic conditions. Together with our cohort of 14 patients, sixty neonatal-onset CCHS cases were used for further analyses. Full phenotypic and genotypic details for the 46 cases in the literature can be found in Table 2.
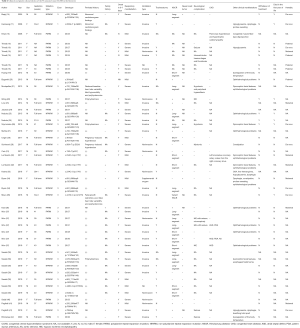
Full table
Overall there were 35 males and 25 females (sex ratio, 1.4). About 20% (18.2%, 10/55) of the patients were born prematurely with the youngest gestational age of 26 weeks. Nearly half (46.2%, 18/39) of the patients had abnormal family history. Polyhydramnios during fetal life occurred in 21.3% (10/47) of the patients. Over 90% (93.2%, 55/59) of the patients manifested symptoms of hypoventilation in the first week of life. About three quarters (43/58) of the patients received invasive mechanical ventilation following intubation initially, 15.5% (9/58) received noninvasive ventilation and 10.3% (6/58) of the patients could live without oxygen or only needed supplemental oxygen via nasal cannula. Twenty-two (41.5%, 22/53) patients performed tracheostomy for airway purposes. Fourteen patients (23.3%) were classified as mild-CCHS and the rest 46 (76.7%) patients were severe-CCHS. Compared with mild-CCHS group, patients in severe-CCHS group presented a higher predisposition to early-onset respiratory manifestation (P=0.002), long-segment HSCR (P=0.046), PARM genotypes (P=0.015) and de novo PHOX2B variants (P=0.003). Parents tend to make a choice of withdrawn (P=0.016) and a higher mortality in the first year of life occurred in severe-CCHS group (Table 3). Gastrointestinal manifestations were seen in 71.7% (38/53) patients. Among them, 30 patients were diagnosed HSCR (12 cases with long-segment-HSCR, 9 cases with short-segment-HSCR, and 9 cases with unknown length of the aganglionic tract), 2 were diagnosed variant HSCR, other 6 patients had dysphagia, abdominal distension and/or persistent constipation. Approximately twofold more males than females were affected by HSCR/variant HSCR (75.8% vs. 35%, P=0.003). Neural crest tumor occurred in 9.1% (4/44) patients. About a quarter (23.7%, 9/38) of the patients were identified as having abnormal cardiac anatomy. No obvious cardiac arrhythmias were reported in this series. Neurological complications such as seizure were observed in 34.1% (15/44) of the patients. Other conditions affecting minority CCHS subjects included: ophthalmological problems (11 patients), dysmorphic facial features (5 patients), hypoglycemia (4 patients), profuse sweating (2 patients), dysregulation of the body temperature (2 patients). The limited available data showed that 35.3% (18/51) of the parents chose to withdraw of treatment and 39.4% (13/33) of the patients were deceased in the first year after birth.
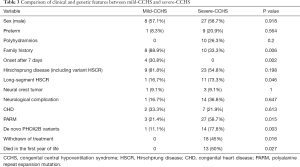
Full table
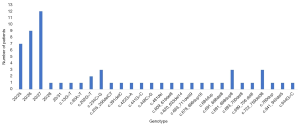
Analysis of PHOX2B gene revealed small alanine expansions (range of +5 to +7 alanines were the most frequent mutations identified in this series (Figure 1). Half of the patients had PARMs in PHOX2B (seven with 25 PARM, nine with 26 PARM, twelve with 27 PARM, one with 28 PARM and one with 31 PARM) and the other half patients had 23 distinct NPARMs including 14 frameshift mutations, 5 missense mutations, 2 nonsense mutations, and 2 readthrough mutations. Four mutations located in exon 1, two located in exon 2 and 17 located in exon 3. Heredity information were available in 27 cases and 14 of them showed a familiar pattern, the rest represented sporadic mutations.
Genotype-phenotype correlation was performed in these 60 neonatal cases (Table 4). All cases carrying 26 or more PARMs and the majority of cases carrying NPARMs presented as severe-CCHS. A higher incidence of HSCR was found in cases carrying larger PARMs and NPARMs. Among PARMs group, no reports of HSCR occurring in cases with the 20/25 genotype and only rare (22.2%) reports with the 20/26 genotype. While HSCR occurred more frequently in subjects with the 20/27 (54.5%) and more PARMs (100%) genotype. There was no significant difference between patients with PARMs and NPARMs regarding sex ratio, preterm birth, polyhydramnios, family history, onset age, neural crest tumor, neurological complication, cardiac anatomy and mortality in the first year of life. However, patients in the PARMs group presented with a higher frequency of severe CCHS (90.0% in the PARMs group vs. 63.3% in the NPARMs group, P=0.015) and less HSCR (43.5% in the PARMs group vs. 73.3% in the NPARMs group, P=0.028).
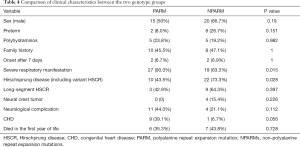
Full table
Discussion
CCHS is a rare disorder characterized by abnormal ventilatory response to hypoxia and hypercapnia, often associated with other autonomic nervous system dysfunctions. Clinical diagnosis of CCHS is based on the presence of central alveolar hypoventilation in the absence of primary pulmonary, cardiac, neuromuscular, or neurologic disease. However, diagnosis is confounded in a critically ill neonate when phenotype is not typical or when the patient is complicated with other conditions predispose to respiratory insufficiency, which is further accentuated in premature newborns, as premature infants often have problems of recurrent central apneas and feeding intolerance (14). Diagnosing CCHS in NICUs for critically ill babies is challenging and molecular analysis may help to diagnose this disorder in this age group.
In this study, we gave the first comprehensive report on 14 Chinese CCHS neonates and reviewed published data on 46 neonatal cases with genetic diagnosis. It includes the largest number of neonatal-onset CCHS reported to date with PHOX2B mutations.
Prenatal/intrapartum history is essential to diagnosis in newborn area. It is noteworthy that, polyhydramnios was observed in 21.3% (10/47) of the patients, higher than reported (3.3%) (2), together with high occurrence of postnatal dysphagia, we speculate the complication of polyhydramnios might be derived from a dysfunction of swallowing control. Abnormal family history including affected patients, abnormal miscarriages and early death was observed in 46.2% (18/39) of the patients. In addition, a few patients presented decreased fetal movements or poor fetal heart rate variability in fetal period. These findings would provide clues for the diagnosis and they emphasized the need for a careful review of the neonates’ history.
CCHS patients suffer a broad spectrum of severity. In the current cohort, about three quarters cases presented as severe-CCHS. Compared with mild-CCHS group, neonates in severe-CCHS group may manifest hypoventilation earlier, be complicated with longer segment HSCR. They may predispose to harbor de novo PHOX2B variants. And their parents tend to choose withdrawn of life thus a higher mortality may occur in the first year of life. Correlations between PHOX2B genotype and CCHS phenotype have been described before. Patients carrying more PARMs or NPARMs appear to produce severer phenotypes with continuous ventilatory support, HSCR with extensive gut involvement, and an increased tumor risk (46). However, several recent studies have identified novel NPARMs in CCHS patients who have a milder clinical presentation and delayed diagnosis, suggesting that NPARMs may have widely variable phenotypic expressivity which was associated with the type and location of the mutation (31,32). Our results revealed that HSCR was indeed more prevalent among NPARMs subjects than among those with PARMs, though no evident correlation between genotype and the extent of HSCR was observed owing to the small sample size. However, a higher proportion of severe-CCHS patients was observed in the PARMs group which was different from previous reports. There are two possible explanations. First, patients with 20/26 PARMs and more PARMs account for majority of this group who present severe hypoventilation extent in neonatal period. Second, patients with NPARMs can present with a diverse range of respiratory phenotypes. We identified 14 mild-CCHS patients from the 60 neonates, apart from 3 patients with 20/25 PARM, we found other 8 NPARMs (c.234C>G, c.255_256delCT, c.391delC, c.448C>G, c.481del, c.663_711del49, c.691_698dup8, c.944G>C) in 11 patients appear to be associated with the mild-CCHS. Among the 11 patients, nine of them had HSCR and another one had neural crest tumor. So, it is currently difficult to assess severity and prognosis based on specific NPARM genotype. In the future, more research is needed on patients with NPARMs to further understand and characterize the variety of associated clinical presentations and elucidate the underlying molecular mechanism.
Neural crest tumors are more frequently diagnosed among CCHS patients over 1 year of age (12). Four patients harboring frameshift PHOX2B mutations were identified having tumor in this study. Interestingly, there was no evidence of tumor in one of the babies through initial abdominal ultrasound screening. While measurements of the catecholamines’ metabolites strongly suggested neuroblastoma and then CT scans were performed to confirm the diagnosis (26). These findings emphasize the need of metabolites tests and CT scans in CCHS neonates especially those patients with frameshift PHOX2B mutations who are at very high risk for developing tumors.
Recurrent episodes of apnea and cyanosis in a newborn should be distinguished from seizure which is a common neurological condition in NICU. Neurological complications were observed in 34.1% (15/44) CCHS neonates, and about half of them presented as seizure. It is unclear whether this is due to hypoxemia or a direct result of the primary neurologic problem associated with CCHS. Thus CCHS should be suspected by neonatologists when confronted with seizure in NICU.
The association between cardiovascular defects and mutations in PHOX2B had been previously assessed by Lombardo et al. (47). Congenital heart disease (CHD) was found in 30% of CCHS patients which was higher than general population (0.8%). The majority of patients had anomalies involving the proximal aortic arch and/or proximal coronary arteries. Similar prevalence of CHD in our cohort was observed (23.7%, 9/38) but anomalies including patent ductus arteriosus and atrial septal defect appears to be more common. In the previous study, five of seven patients identified with CHD had either NPARMs or whole-gene deletion, while in the current cohort, more patients with PARMs were identified with CHD, though without significantly difference (P=0.055). Considering the small sample size of both studies, we suggest a large-scale, multicenter study in this field.
Pertaining to genetic findings, higher frequency of NPARMs (50%) was identified in this group of neonates than previous data (10%). This may be explained by the previous finding that patients carrying shorter PARMs like 20/24, 20/25 PARM may have a mild phenotypic consequence who exhibit hypoventilation in late childhood or adulthood, while our study was specific to newborns (5). Triplet expansion of 27 nucleotides (20/27) was the most frequent mutation (20.0%) identified in our cohort. Thus far, nearly 200 cases associated with NPARMs have been reported, with about 100 unique variants identified (33). Here, we reported a novel NPARM of PHOX2B (c.684dup) which was predicted to cause frameshift and produce prolonged protein in a preterm neonate with severe CCHS and HSCR. This finding contributes to accumulating data on the genotype-phenotype correlations and providing useful information for clinical practice.
CCHS is typically inherited in an autosomal dominant pattern, and most mutations occur de novo (4). Recent evidence indicates that a subset is inherited from parents carrying somatic or germline mosaicism for these PHOX2B mutations, thus the prevalence of de novo mutations may be overestimated (48). In this study, fourteen cases showed a familiar pattern, thirteen represented sporadic mutations while in 33 cases familiarity was not reported. Among them, CCHS was observed in one pair of Chinese siblings with the same CCHS-causing PHOX2B 20/26 PARM, as this 20/26 genotype is rare, with about 200 cases identified and reported worldwide (6), the recurrence of the same disease in this family strongly indicated inheritance of the PHOX2B mutation from an asymptomatic parent. However, this 20/26 mutation could not be found on testing PHOX2B gene in the parents’ blood samples thus germline mosaicism had been hypothesized to explain sibling recurrence for CCHS in this family which deserved further investigations (49). These findings highlighted the importance of family planning and genetic counseling for families of a PHOX2B mutation confirmed proband.
The current study also indicates that there are ethical problems in decision making in this group of neonates, and death occurrence is highly related to ethical decisions, especially in China. Mortality rates of 8% to 38% have been documented in various CCHS patient cohorts (50). In this cohort, available data showed that 35.3% (18/51) of the parents chose to withdraw of treatment and 39.4% (13/33) of the patients were deceased in the first year after birth. A striking finding was that nearly all the Chinese parents (92.9%, 13/14) elected withdrawal of treatment. We speculate that most of them would be died as cases with neonatal-onset CCHS are in such a critical condition. Long-term outcome is the issue that most concerns parents. Babies with CCHS are routinely managed with a tracheostomy and ventilation, parents do not want their children to suffer with a lifetime of ventilation and they doubt their ability to care for them at home. They are also concerned about the impact of this child’s illness on other children and their family. At the same time, the medical and homecare requirements of CCHS children impose significant annual healthcare costs that few family incomes would cover without financial support in China. On the other side, neonatologists are generally pessimistic about medical outcomes and they tend to give parents the option to withdrawal of life support in this situation. In developed countries such as America, France and Japan, specialized centers were established to promote CCHS care and research. With advances in early diagnosis and home ventilation, CCHS patients are surviving into adulthood and reporting good quality of life (51). From a long-term follow-up of 196 children with CCHS in North America and Europe, vast majority (88%) of parents stated that things got better with time (52). As a developing country, China has much work to do to help healthcare professionals and parents gain confidence and comfort in managing CCHS such as providing optimal training and education.
Study limitations
As the present study was a single-center report with literature reviewing combination, methodological issues like skewed patient recruitment may limit our conclusions. First, this survey only targeted those CCHS neonates with genetic diagnosis, we may have missed relevant information on CCHS cases without genetic data. Secondly, for cases with genetic data, we could not obtain relevant clinical information that were not reported in the literatures, thus some phenotypic characteristics may have been underrepresented in this study. Considering sampling bias, these results should be confirmed in more patients. At the same time, large-scale follow-up studies are required for further information on disease progression.
Conclusions
Here, we report 14 additional neonatal-onset CCHS cases and provide an extensive overview of a series of 60 neonates with PHOX2B pathogenic variants. This series reveals that (I) about three quarters neonatal cases manifest as severe-CCHS and 60.4% of the cases are accompanied by HSCR which is more observed in males and in NPARMs cases. (II) NPARMs occur more frequently in this early onset group with highly variable respiratory features and some of the genotypes are more likely to be associated with mild phenotypes. (III) Molecular testing is crucial for timely diagnosis and genetic counseling. These findings may have positive effect on early diagnosis and management of CCHS in newborn area.
Acknowledgments
The authors thank all the patients and their families for their participation in this study.
Funding: This study was supported by grants from National Natural Science Foundation of China [81671502], and Shanghai Municipal Commission of Health and Family Planning [20174Y0060].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-303
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-303
Peer Review File: Available at http://dx.doi.org/10.21037/tp-20-303
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-303). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the Children’s Hospital of Fudan University [2015 (no. 169)] and adhered to the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from patients’ guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trang H, Dehan M, Beaufils F, et al. The French Congenital Central Hypoventilation Syndrome Registry: general data, phenotype, and genotype. Chest 2005;127:72-9. [Crossref] [PubMed]
- Shimokaze T, Sasaki A, Meguro T, et al. Genotype-phenotype relationship in Japanese patients with congenital central hypoventilation syndrome. J Hum Genet 2015;60:473-7. [Crossref] [PubMed]
- Trang H, Samuels M, Ceccherini I, et al. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J Rare Dis 2020;15:252. [Crossref] [PubMed]
- Berry-Kravis EM, Zhou L, Rand CM, et al. Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype. Am J Respir Crit Care Med 2006;174:1139-44. [Crossref] [PubMed]
- Bishara J, Keens TG, Perez IA. The genetics of congenital central hypoventilation syndrome: clinical implications. Appl Clin Genet 2018;11:135-44. [Crossref] [PubMed]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, et al. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 2010;181:626-44. [Crossref] [PubMed]
- Sasaki A, Kanai M, Kijima K, et al. Molecular analysis of congenital central hypoventilation syndrome. Hum Genet 2003;114:22-6. [Crossref] [PubMed]
- Weese-Mayer DE, Rand CM, Berry-Kravis EM, et al. Congenital central hypoventilation syndrome from past to future: model for translational and transitional autonomic medicine. Pediatr Pulmonol 2009;44:521-35. [Crossref] [PubMed]
- Wang Y, He XY, Yang Y, et al. Congenital central hypoventilation syndrome, report of three cases. Zhonghua Er Ke Za Zhi 2013;51:852-5. [PubMed]
- Broch A, Trang H, Montalva L, et al. Congenital central hypoventilation syndrome and Hirschsprung disease: A retrospective review of the French National Registry Center on 33 cases. J Pediatr Surg 2019;54:2325-30. [Crossref] [PubMed]
- Hennewig U, Hadzik B, Vogel M, et al. Congenital central hypoventilation syndrome with hyperinsulinism in a preterm infant. J Hum Genet 2008;53:573-7. [Crossref] [PubMed]
- Trochet D, O'Brien LM, Gozal D, et al. PHOX2B genotype allows for prediction of tumor risk in congenital central hypoventilation syndrome. Am J Hum Genet 2005;76:421-6. [Crossref] [PubMed]
- Hung CC, Su YN, Tsao PN, et al. Unequal crossover recombination - population screening for PHOX2B gene polyalanine polymorphism using CE. Electrophoresis 2007;28:894-9. [Crossref] [PubMed]
- Bajaj R, Smith J, Trochet D, et al. Congenital central hypoventilation syndrome and Hirschsprung's disease in an extremely preterm infant. Pediatrics 2005;115:e737-8. [Crossref] [PubMed]
- Khan A, Sarnat HB, Spaetgens R. Congenital muscle fiber-type disproportion in a patient with congenital central hypoventilation syndrome due to PHOX2B mutations. J Child Neurol 2008;23:829-31. [Crossref] [PubMed]
- Trivedi A, Waters K, Suresh S, et al. Congenital central hypoventilation syndrome: four families. Sleep Breath 2011;15:785-9. [Crossref] [PubMed]
- Jones KL, Pivnick EK, Hines-Dowell S, et al. A triple threat: Down syndrome, congenital central hypoventilation syndrome, and Hirschsprung disease. Pediatrics 2012;130:e1382-4. [Crossref] [PubMed]
- Meguro T, Yoshida Y, Hayashi M, et al. Inheritance of polyalanine expansion mutation of PHOX2B in congenital central hypoventilation syndrome. J Hum Genet 2012;57:335-7. [Crossref] [PubMed]
- Huang LC, Chang JH, Wang NL. Congenital central hypoventilation syndrome in a full-term baby presenting with repeated extubation failure. Pediatr Neonatol 2012;53:72-4. [Crossref] [PubMed]
- Bygarski E, Paterson M, Lemire EG. Extreme intra-familial variability of congenital central hypoventilation syndrome: a case series. J Med Case Rep 2013;7:117. [Crossref] [PubMed]
- De Montpellier S, Sznajer Y, Amiel J, et al. An unusual cause of fetal hypomobility: congenital central hypoventilation syndrome associated with hirschsprung disease. Eur J Pediatr 2014;173:1607-9. [Crossref] [PubMed]
- Wang TC, Su YN, Lai MC. PHOX2B mutation in a Taiwanese newborn with congenital central hypoventilation syndrome. Pediatr Neonatol 2014;55:68-70. [Crossref] [PubMed]
- Low KJ, Turnbull AR, Smith KR, et al. A case of congenital central hypoventilation syndrome in a three-generation family with non-polyalanine repeat PHOX2B mutation. Pediatr Pulmonol 2014;49:E140-3. [Crossref] [PubMed]
- Jaiyeola P, El-Metwally D, Viscardi R, et al. Congenital hypoventilation syndrome and Hirschsprung's disease - Haddad syndrome: A neonatal case presentation. J Neonatal Perinatal Med 2015;8:165-8. [Crossref] [PubMed]
- Nobuta H, Cilio MR, Danhaive O, et al. Dysregulation of locus coeruleus development in congenital central hypoventilation syndrome. Acta Neuropathol 2015;130:171-83. [Crossref] [PubMed]
- Szymońska I, Borgenvik TL, Karlsvik TM, et al. Novel mutation-deletion in the PHOX2B gene of the patient diagnosed with Neuroblastoma, Hirschsprung's Disease, and Congenital Central Hypoventilation Syndrome (NB-HSCR-CCHS) Cluster. J Genet Syndr Gene Ther 2015;6:269. [PubMed]
- Mehta VJ, Ling JJ, Martinez EG, et al. Congenital Tonic Pupils Associated With Congenital Central Hypoventilation Syndrome and Hirschsprung Disease. J Neuroophthalmol 2016;36:414-6. [Crossref] [PubMed]
- Unger SA, Guillot M, Urquhart DS. A Case of "Abnormally Abnormal" Hypoxic Ventilatory Responses: A Novel NPARM PHOX 2B Gene Mutation. J Clin Sleep Med 2017;13:1013-5. [Crossref] [PubMed]
- Schirwani S, Pysden K, Chetcuti P, et al. Carbamazepine Improves Apneic Episodes in Congenital Central Hypoventilation Syndrome (CCHS) With a Novel PHOX2B Exon 1 Missense Mutation. J Clin Sleep Med 2017;13:1359-62. [Crossref] [PubMed]
- Al Dakhoul S. Haddad syndrome novel association with BRAF mutation. J Neonatal Perinatal Med 2017;10:455-7. [Crossref] [PubMed]
- Cain JT, Kim DI, Quast M, et al. Nonsense pathogenic variants in exon 1 of PHOX2B lead to translational reinitiation in congenital central hypoventilation syndrome. Am J Med Genet 2017;173:1200-7. [Crossref] [PubMed]
- Lombardo RC, Kramer E, Cnota JF, et al. Variable phenotype in a novel mutation in PHOX2B. Am J Med Genet 2017;173:1705-9. [Crossref] [PubMed]
- Katwa U, D'Gama AM, Qualls AE, et al. Atypical presentations associated with non-polyalanine repeat PHOX2B mutations. Am J Med Genet 2018;176:1627-31. [Crossref] [PubMed]
- Byers HM, Chen M, Gelfand AS, et al. Expanding the phenotype of congenital central hypoventilation syndrome impacts management decisions. Am J Med Genet 2018;176:1398-404. [Crossref] [PubMed]
- Miura Y, Watanabe T, Uchida T, et al. A novel PHOX2B gene mutation in an extremely low birth weight infant with congenital central hypoventilation syndrome and variant Hirschsprung's disease. Eur J Med Genet 2019;62:103541 [Crossref] [PubMed]
- Kasi AS, Kun SS, Keens TG, et al. Adult With PHOX2B Mutation and Late-Onset Congenital Central Hypoventilation Syndrome. J Clin Sleep Med 2018;14:2079-81. [Crossref] [PubMed]
- Woo HY, Oh C, Han JW, et al. Clinical features of children with Haddad syndrome: A single-center experience. J Pediatr Surg 2020;55:387-92. [Crossref] [PubMed]
- Sasaki A, Kishikawa Y, Imaji R, et al. Novel PHOX2B mutations in congenital central hypoventilation syndrome. Pediatr Int 2019;61:393-6. [Crossref] [PubMed]
- Sivan Y, Zhou A, Jennings LJ, et al. Congenital central hypoventilation syndrome: Severe disease caused by co-occurrence of two PHOX2B variants inherited separately from asymptomatic family members. Am J Med Genet 2019;179:503-6. [Crossref] [PubMed]
- Saddi V, Teng A, Thambipillay G, et al. Nasal mask average volume-assured pressure support in an infant with congenital central hypoventilation syndrome. Respirol Case Rep 2019;7:e00448 [Crossref] [PubMed]
- Paglietti MG, Cherchi C, Porcaro F, et al. Two novel mutations in exon 3 of PHOX2B gene: think about congenital central hypoventilation syndrome in patients with Hirschsprung disease. Ital J Pediatr 2019;45:49. [Crossref] [PubMed]
- Binmanee A, Alfadhel A, Alzamil N, et al. Congenital Central Hypoventilation Syndrome Presenting with Seizures. Cureus 2020;12:e6680 [Crossref] [PubMed]
- Kincaid PK, Dietrich RB, Pais MJ. Pediatric case of the day. Neurocristopathy (Ondine-Hirschsprung syndrome). Radiographics. 1994;14:1139-43. [Crossref] [PubMed]
- Sandoval RL, Zaconeta CM, Margotto PR, et al. Congenital central hypoventilation syndrome associated with Hirschsprung's Disease: case report and literature review. Rev Paul Pediatr 2016;34:374-8. [Crossref] [PubMed]
- Sinclair R, Teng A, Jonas C, et al. Congenital central hypoventilation syndrome: A pictorial demonstration of absent electrical diaphragmatic activity using non-invasive neurally adjusted ventilatory assist. J Paediatr Child Health 2018;54:200-2. [Crossref] [PubMed]
- Weese-Mayer DE, Rand CM, Zhou A, et al. Congenital central hypoventilation syndrome: a bedside-to-bench success story for advancing early diagnosis and treatment and improved survival and quality of life. Pediatr Res 2017;81:192-201. [Crossref] [PubMed]
- Lombardo RC, Porollo A, Cnota JF, et al. Congenital heart disease and aortic arch variants associated with mutation in PHOX2B. Genet Med 2018;20:1538-43. [Crossref] [PubMed]
- Trang H, Brunet JF, Rohrer H, et al. Proceedings of the fourth international conference on central hypoventilation. Orphanet J Rare Dis 2014;9:194. [Crossref] [PubMed]
- Rand CM, Yu M, Jennings LJ, et al. Germline mosaicism of PHOX2B mutation accounts for familial recurrence of congenital central hypoventilation syndrome (CCHS). Am J Med Genet 2012;158A:2297-301. [Crossref] [PubMed]
- Healy F, Marcus CL. Congenital central hypoventilation syndrome in children. Paediatr Respir Rev 2011;12:253-63. [Crossref] [PubMed]
- Verkaeren E, Brion A, Hurbault A, et al. Health-related quality of life in young adults with congenital central hypoventilation syndrome due to PHOX2B mutations: a cross-sectional study. Respir Res 2015;16:80. [Crossref] [PubMed]
- Vanderlaan M, Holbrook CR, Wang M, et al. Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol 2004;37:217-29. [Crossref] [PubMed]


