
Multisystem inflammatory syndrome in children during the coronavirus disease 2019 (COVID-19) pandemic: a systematic review of published case studies
Introduction
Since the global pandemic of coronavirus disease 2019 (COVID-19), the morbidity and mortality of COVID-19 in children have been concerned by researchers closely. As reported from previous studies, the clinical manifestations of children with COVID-19 were milder than that of adults, and severe cases and deaths were rare (1,2). On April 7th, 2020, a case study from the United States reported a 6-month-old girl admitted and diagnosed with Kawasaki disease (KD) whose reverse-transcriptase polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 was positive (3). On April 27th, the Guardian published an article informing 12 cases in England, which was the first alert in the European media. Since then, more and more similar cases have been published. Two case series published in the Lancet, one from the United Kingdom and another from Italy have provided more evidence (4,5). These cases of children with KD-like manifestations have increased significantly in the local area, most with evidence of SARS-CoV-2 infection. The National Health Service (NHS) issued a warning in late April that a multisystem inflammatory syndrome which could be linked to COVID-19 has led to a surge in the number of children requiring intensive care unit (ICU) admission, and its characteristics generally overlap with toxic shock syndrome (TSS) and atypical KD. In mid-May, the Centers for Disease Control and Prevention (CDC) launched an alert to identify Multisystem Inflammatory Syndrome in Children (MIS-C) and proposed a case definition (6). Other than MIS-C, the condition has also been termed pediatric multisystem inflammatory syndrome (PMIS), pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), pediatric hyperinflammatory syndrome, Kawasaki-like disease, etc.
For now, the overall incidence of MIS-C is low. Case studies as observational studies, play a crucial role in low-incidence diseases like MIS-C, which preliminarily define the risk factors of the disease and describe its clinical manifestations and outcomes. However, case studies also have some shortcomings such as small sample size and cases mostly originate from single center. Although several reporting guidance and quality appraisal tools of case studies, such as IHE and CARE checklists, have been published, improving the standards and quality of reported case studies still remains quite a challenge (7). Therefore, we systematically retrieved case studies on patients with MIS-C to clarify its clinical features, treatments and outcomes. In addition, we used IHE and CARE tools to assess the published case studies, thus analyzing their risk of bias and reporting quality. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tp-20-188).
Methods
Search strategy
We searched the following electronic databases: MEDLINE (via PubMed), Web of Science, CBM (China Biology Medicine disc) and China National Knowledge Infrastructure (CNKI) from their inception to June 17, 2020. The main search terms used were “COVID-19”, “SARS-CoV-2”, “Novel coronavirus”, “coronavirus disease 2019”, “multisystem inflammatory syndrome in children”, “MIS-C”, “Kawasaki disease”, “Kawasaki-like disease”, “PMIS” and their derivatives (the full search strategy can be found in Supplementary material 1). We also searched the reference lists of all included publications for potential studies.
Inclusion and exclusion criteria
We included studies that met the following criteria: (I) population: children and adolescents (age <21 years) with multisystem inflammatory condition [with symptoms of multisystem inflammation or meeting the case definitions of MIS-C given by CDC or PIMS-TS given by the Royal College of Paediatrics and Child Health (RCPCH)] during the pandemic of COVID-19 (published cases of young adults over 21 years of age were also included, if any); (II) study design: studies that contain adequate information of the target population, including case reports, case series, and other studies like cohort studies, letters and correspondences with case reports; (III) outcomes: demographic characteristic, clinical symptoms, laboratory and imaging findings, therapeutic managements and outcomes of children with multisystem inflammatory condition.
We excluded: (I) animal studies, in vitro experiments and epidemiological studies; (II) studies in which data on the target population cannot be extracted; (III) duplicates; (IV) studies not published in English or Chinese; (V) studies with no access to full text; (VI) reviews, systematic reviews, comments, and conference abstracts.
Study selection
According to the search strategy established before, all retrieved studies were screened independently by two reviewers (W Li and Y Tang) after eliminating duplicates. Any discrepancies were resolved by discussion, consulting a third reviewer (E Liu) if necessary. First, the titles and abstracts were screened to exclude irrelevant studies, using the bibliographic software Endnote. Second, potential eligible studies were assessed by reviewing the full texts to make sure they all met the inclusion criteria. All the reasons for exclusion of ineligible studies were recorded, and the process of study selection was documented using a PRISMA flow diagram (8).
Data extraction
Two reviewers (W Li and Y Tang) independently extracted data of the included studies with a standard data collection form using a Microsoft Excel database. Any disagreement was resolved by discussion and consensus.
The following data were collected from each study: (I) basic information (first author name, journal, study design, date of publication, country, city, sample size, age and gender of included patients, terms in the title); (II) clinical manifestations; (III) laboratory and imaging findings [chest X-ray, computed tomography (CT) scan, echocardiography findings, etc.]; (IV) treatments [intravenous immunoglobulin (IVIG), steroids, immunomodulators, antibiotics, etc.] and outcomes (hospitalization, ICU admission, or death). For clinical manifestations, imaging findings, treatments and outcomes, we extracted the percentages of patients; for laboratory findings, we extracted the absolute values (means or medians) of each test.
Statistical analysis
Data of case series with at least 10 patients were shown as ranges of percentages or absolute values in the results part, from the lowest to the highest level. For case reports and case series with less than 10 patients, we extracted important clinical data and selectively described its significant items. Besides, we combined the percentages (clinical manifestations, imaging findings, treatments and outcomes) using STATA version 15. We only presented the range of the absolute values (laboratory findings) considering its great statistical and clinical heterogeneity.
Risk of bias assessment
Two reviewers (W Li and Y Tang) assessed the risk of bias of included case studies independently. Study quality of case series will be assessed using the quality appraisal checklist developed by IHE (9). Each item will be assessed to be yes, partial or no, which represented clear description, unclear description or not reported, respectively. Meanwhile, all case reports and case series studies will be assessed by CARE checklist (10), a reporting guideline for case reports of one or more patients, using the same criteria as IHE. Cohort studies were deemed as case series and assessed as described above. For other types of included studies such as letter and correspondence, we did not assess their quality as they were not applicable to these two tools.
As COVID-19 pandemic is an unprecedented public health emergency, this study was not registered in order to speed up the process.
Results
The literature search yielded 48 articles (Figure 1). After removing two duplicates, titles and abstracts were screened for 46 articles, and of those, 24 articles were excluded. Twenty-two full texts were assessed and met the inclusion criteria. Additional two records were identified through the reference lists of included studies, resulting in a total of 24 studies finally included.
Study characteristics and demographic features
The publication time was between April 7th and June 9th, 2020. Ten originated from the United States, two from India (3,11-21), and the rest were mainly from Europe, such as the United Kingdom (5,22,23), France (24-29), and Italy (4). Thirteen case series were enrolled (4,5,13-16,22-26,28,29), including one prospectively designed (28), and two published in the form of letter or correspondence (5,16). Nine studies had patients of ≥10 (4,14,16,22-26,28). In total, 270 MIS-C cases were reported, and the male to female ratio was close to 1:1 [134/136], with the median age of onset being 7.3 to 11 years (range, 0.3 to 20 years), which is older than patients with KD (normally less than 5 years old). A case series from London showed higher proportion of patients of African ancestry [6/8] (5), and another study revealed that 57% of patients had at least one parent originating from Africa (28), which indicated that genetic factor might play a role in the pathogenesis of MIS-C. As MIS-C is a newly emerged syndrome, a variety of terms were seen in different articles, including MIS-C, PMIS-TS, PIMS, KD-like syndrome, etc. (Table 1).
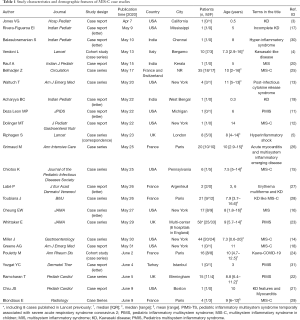
Full table
Symptoms, clinical syndrome and evidence of SARS-CoV-2 infection
As shown in Table 2, all patients had fever (100%), and 84% (95% CI: 77%, 90%) of them had gastrointestinal symptoms like abdominal pain, vomiting, and diarrhea. One prospective study with 21 patients reported that all patients had gastrointestinal symptoms, which commonly occurred early in the course of disease before the onset of symptoms of KD (28). However, respiratory symptoms like cough and rhinorrhea, were relatively infrequent (12–34%). Forty-three percent to 100% of patients had shock, the vast majority of whom were cardiogenetic. The proportion of patients who meet the diagnostic criteria of Kawasaki Disease Shock Syndrome (KDSS), KD, and incomplete Kawasaki disease (IKD) were 44% to 57%, 22% to 63%, and 22% to 53%, respectively. One study reported that 56% of the patients met the diagnosis of macrophage activation syndrome (MAS) (4). Symptoms mimicking KD were common, such as rash (50–81%), conjunctivitis (30–94%), oral mucosal changes (25–87%), limb changes such as redness and induration (16–68%) and lymphadenopathy (10–60%). Moreover, 26% to 56% of patients had neurological symptoms including headache, confusion, and meningeal signs.
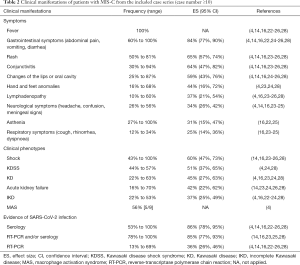
Full table
In general, 78% to 100% of patients had evidence of SARS-CoV-2 infection, in which 86% (95% CI: 78%, 95%) of patients had positive serologic testing for SARS-CoV-2, whereas 36% (95% CI: 26%, 46%) of patients tested positive by RT-PCR.
Laboratory findings
Increased inflammatory markers were widely observed. Leukocyte count elevated [(10.8–17.4) ×109/L] with mainly neutrophils [(9.2–13.6) ×109/L] suggested inflammation, along with decreased lymphocytes count [(0.8–1.2) ×109/L]. C-reactive protein (CRP) (146.5–253 mg/L) and procalcitonin (PCT) (21.7–46 ng/mL) increased tremendously. The ferritin was in the range of 558 to 1,176 ng/mL. One study showed that 86% of patients had abnormal ferritin level, and it was one of the factors that distinguished between severe and non-severe cases (1,760 vs. 295 µg/L, P=0.003) (24). High levels of erythrocyte sedimentation rate (ESR) (59–75 mm/h) and interleukin-6 (IL-6) (135–226.3 pg/mL) were also common. In terms of liver and kidney function, decreased albumin (21–37 g/L) and slightly increased creatinine (59–174 µmol/L) were observed, and a small number of patients showed slightly increased alanine transaminase (ALT). Myocardial injury was quite common, 55% to 100% of patients had increased troponin, with a level of 0.045 to 269 µg/L. Brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) were also increased, ranging from 3,354 to 5,743 pg/mL and 788 to 41,484 pg/mL, respectively. Besides, a significant increase in D-dimer (2,060–5,284 ng/mL) and decreased sodium levels (130–131 mmol/L) were noticed (Table 3).
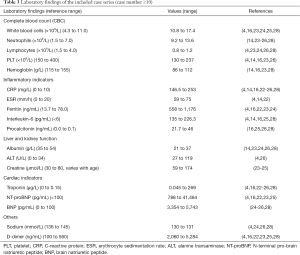
Full table
Imaging findings
Thirty-one percent to 50% of patients showed abnormal results of chest imaging (chest X-ray or CT) such as ground-glass shadows, local patchy shadows, or interstitial abnormalities that indicated pneumonia. The most common symptom on echocardiography was myocarditis, with 61% (95% CI: 29%, 93%) of children involved. Besides, left ventricular ejection fraction (LVEF) reduction was noted in 54% (95% CI: 39%, 69%) of patients, most of them had LVEF less than 50%, and the lowest was only 10% (28). Pericardial effusion and coronary artery dilatation were found in 34% (95% CI: 17%, 52%) and 23% (95% CI: 8%, 39%) of patients, respectively. Coronary aneurysms occurred less frequently, found in 11 patients from four studies [11/100] (4,16,22,23), including two patients with giant aneurysms (z score >10) (23). One study also identified pericarditis in 4 out of 16 patients (Table 4).
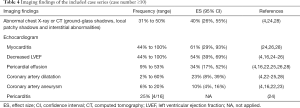
Full table
Treatment and outcomes
We cannot estimate the effect of one single therapy due to the fact that most patients received multiple medications and therapies. In general, IVIG and anticoagulants were commonly used. As shown in Table 5, 78% (95% CI: 70%, 87%) of the patients received IVIG infusion. However, 25% (95% CI: 6%, 45%) of them showed IVIG resistance and required a second use of IVIG, or IVIG added with glucocorticoids. Compared with typical KD patients, the proportion of IVIG resistance in MIS-C patients was significantly higher (10/16 vs. 45/220, P=0.004) (24), and the need for adjunctive steroid treatment also increased significantly (4/19 vs. 8/10, P=0.0045) (4). Seventy percent (95% CI: 50%, 89%) of patients received anticoagulants such as heparin or aspirin, and aspirin was applied in 53% (95% CI: 14%, 93%) of patients, mostly as an anticoagulant, but sometimes at anti-inflammatory doses.
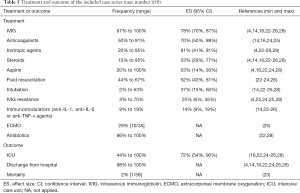
Full table
Furthermore, 53% (95% CI: 29%, 77%) of patients were reported to require steroids. Fourteen percent (95% CI: 9%, 19%) of patients received immunomodulators (such as anti-IL-1, anti-IL-6, anti-TNF-α drugs), of which the most commonly used was IL-1 receptor antagonist Anakinra. Use of inotropic agents (epinephrine, dobutamine, milrinone and norepinephrine) were seen in 61% (95% CI: 41%, 81%) of patients, and fluid resuscitation in 52% (95% CI: 43%, 61%) of patients, due to heart failure or shock. Empirical broad-spectrum antibiotics treatment was common (86–100%), with third-generation cephalosporin always included. One case series with 35 critically ill patients reported that 29% of patients used extracorporeal membrane oxygenation (ECMO), and they successfully extubated from ECMO after a median duration of 4.5 (range, 3–6) days, with improvement of clinical condition (25). The proportion of patients requiring invasive mechanical ventilation such as intubation was 37% (95% CI: 15%, 60%). Despite the fact that 72% (95% CI: 54%, 90%) of the patients were admitted to ICU, almost all of them improved and were discharged home. In all case studies included, only one death was recorded. The patient was a 14-year-old Afro-Caribbean girl who required mechanical ventilation and ECMO support but finally died from a large cerebrovascular infarct. SARS-CoV-2 infection was confirmed after death (5).
Risk of bias
Eleven case series were assessed by IHE checklists, and only 6 of them had an overall score of more than 0.7 (14,23-26,28), representing an acceptable risk of bias (Table 6). It can be seen that there was a lack of detailed description in item 2 (prospective design), item 3 (multi-center), item 11 (blind method), and item 18 (side effect). Attention should be paid to these aspects when conducting case series, for strengthening the study design and reporting quality. Of note, none of these studies mentioned whether they used any reporting checklist.
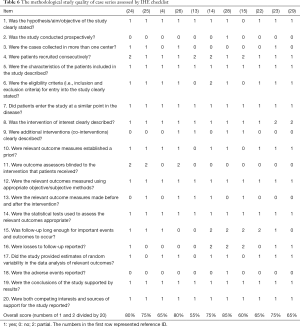
Full table
CARE assessment revealed that most of the studies were poorly reported in the aspect of title and keywords sections (Figure 2). Twelve (50%) studies did not mention ethics committee approval as well as patient informed consent. Significantly, the follow-up part and patient perspective part scored low. Timeline was also not well described except for case report that involved only one patient. In addition, all studies failed to mention the perspectives of patients.
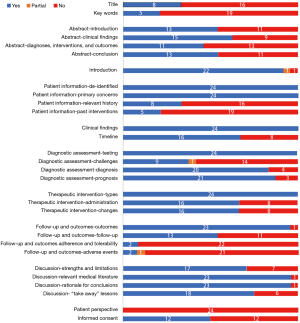
Discussion
As a newly emerged clinical syndrome, MIS-C is characterized by severe symptoms, rapid progression, and unclear pathogenesis. Controlled studies cannot be carried out in the short term, case studies therefore are of great value during public health emergencies. Our case review comprehensively evaluated the clinical features, laboratory findings, imaging findings, treatments, and outcomes of MIS-C patients, aiming to provide reference for identification and treatment of MIS-C for clinicians. Meanwhile, IHE and CARE tools were used to assess the quality of case studies.
Our study showed that, unlike children with COVID-19 (2), respiratory symptoms were not common in patients with MIS-C. In contrast, fever and gastrointestinal symptoms including abdominal pain or vomiting, were the main presenting manifestations. Other symptoms similar to KD and TSS, such as shock, rash, conjunctivitis, oral mucosal changes, limb changes (redness, swelling or induration), and lymphadenopathy, were also common. Most patients had one or more elevated laboratory biomarkers indicating inflammation, such as CRP, PCT, ESR, ferritin, IL-6, and D-dimer, accompanied by neutrophilia and lymphopenia. Myocarditis, decreased LVEF, pericardial effusion and coronary artery dilatation were common on echocardiography, and chest radiograph or CT indicating pneumonia were seen only in a small group of patients. It is thus clear that children with MIS-C had a severe inflammatory response, which involved multiple organs, and the cardiovascular system was the primary target. Several reports of vascular and multisystem inflammatory involvement in adult patients with COVID-19 had been published before (32-34). Since symptoms in patients with MIS-C differ from typical COVID-19, the current incidence of MIS-C is likely to be underestimated.
At present, the management of MIS-C was mostly based on the clinical presentation of KD, KDSS, and TSS. IVIG, anticoagulants and steroids were the main therapies. Empiric antibiotic therapy was also in common use. Considering the complexity and variety of the clinical manifestation, which could present symptoms that mimic TSS or septic shock, empirical use of antibiotics is reasonable until negative bacterial culture results were confirmed. However, guidelines are needed to standardize the use of antibiotics. In general, the majority of patients with MIS-C had a favorable outcome. Although a considerable proportion of patients had experienced a severe course of disease, including the need for endotracheal intubation, and fluid resuscitation or inotropic agents due to circulatory failure. Therefore, we should be aware of the potential occupation of medical resources caused by MIS-C, and pediatricians in high-incidence areas need to prepare with technical and medical resources in advance. Besides, the evidence-based guidelines and systematic reviews published by our research group did not recommend routine use of systemic corticosteroids and IVIG in children with COVID-19 (35-37), but the emergence of MIS-C calls for a re-evaluation of the value of steroids and IVIG. A recent study in adults also indicated that glucocorticoids could reduce the mortality in COVID-19 patients (38).
In the early stage of COVID-19 outbreak, the hypothesis of viral sepsis caused by SARS-CoV-2 was proposed and used to explain the presentation of severe or critically ill patients, particularly those with shock (39). It suggested that viral sepsis should be considered when patients met the diagnostic criteria for sepsis and septic shock and had negative bacteria and fungus culture results. Yet, viral sepsis seems to be inappropriate when applied to MIS-C. Different from severe adult patients with COVID-19, where high viral loads of SARS-CoV-2 were reported, children with MIS-C in our study showed no viraemia of SARS-CoV-2. Besides, our study also verified that the majority of the patients with MIS-C had positive serological evidence, while positive RT-PCR testings were less commonly observed. However, among those who did test positive for RT-PCR, there were possibilities that the testing results were unreliable, as false positive results (identification of viral RNA residues) could sometimes occur. This was further supported by one epidemiological study that suggested the peak incidence of MIS-C occurred one month after the peak of COVID-19 hospitalizations (40). Moreover, as observed in our study, use of IVIG, IL-6 receptor antagonists, and TNF-α receptor antagonists received sound effects, indicating that cytokine storm might play an essential role in the immunopathology of MIS-C. Hence, we hypothesized that MIS-C is not an acute phase response, but a delayed post-infection process that relates to the abnormal immune response after acute infection has passed.
In terms of diagnosis, CDC, World Health Organization (WHO), and RCPCH have proposed relevant case definition (6,41,42). These definitions are slightly different and likely to change as our recognition of the disease is evolving rapidly. Besides, our review found that a variety of terms had been used to describe the syndrome, including MIS-C, PMIS-TS, PIMS-TS, Kawasaki-like disease, hyperinflammatory syndrome, Kawa-COVID-19, etc. Different terms in the literature could lead to confusion and hinder the efficiency of scientific communication. Thus, unified terminology and diagnostic criteria should be established.
Thirteen case series and 11 case reports (including two cohort studies and eight published in the form of letters and correspondence) were included, none of which mentioned the use of any reporting checklists. When assessed by CARE and IHE tools, most studies failed to follow the standard reporting checklist, which we found consistent with a previously published systematic review of COVID-19 case studies in children that used the IHE tool to evaluate the risk of bias of included literature (7). Some items are inapplicable to pediatrics and public health emergencies, such as patient perspective (as it is often parents that communicate with the doctors for their child), intervention adherence and tolerability, and related follow-up items. However, some important items need to be followed for the completeness and transparency for scientific case studies, such as title (state the diagnosis or intervention of primary focus followed by the words “case report”), keywords (identify diagnoses or interventions in this case report), informed consent (provide patients’ consent), and timeline (organize the historical and current information from this episode of care in chronological order).
This study has several limitations. First, considering the great clinical and statistical heterogeneity such as varied time point in a disease course when recording the results, varied test equipments and that most articles showed their results as medians (range) instead of means (SD), we didn’t combine the data of the laboratory findings. Second, there might be repeated statistics since newly emerged diseases like MIS-C always attract the attention of clinicians and researchers; one case may have been reported from different perspectives in another study. Third, data of various studies were presented with a different statistic, mainly including means and medians in most cases. Besides, some studies showed dynamic changes in data in the course of disease, while most studies only provided data on admission, which could lead to inconsistency when comparing data.
Conclusions
Our case review systematically summarized and evaluated the clinical characteristics, treatment, and outcomes of patients with MIS-C. Based on the currently limited data, we concluded that patients with MIS-C are more severe than children with COVID-19, with fever and gastrointestinal symptoms as the primary symptoms and multisystem involvement, particularly cardiovascular system. Most children had a favorable outcome after multidisciplinary care, but a longer follow-up to monitor the potential sequelae and further researches to explore the possible causal correlation between MIS-C and SARS-CoV-2 are urgently needed. It is worth noting that case studies can provide us with the latest and reliable medical data under public health emergencies. Still, most of the case studies did not follow the standard reporting checklist, so that they failed to get higher scores in the risk of bias assessment.
Acknowledgments
Funding: This study was funded by National Clinical Research Center for Child Health and Disorders (Children’s Hospital of Chongqing Medical University, Chongqing, China) (NCRCCHD-2020-EP-01); the fourth batch of “Special Project of Science and Technology for Emergency Response to COVID-19” of Chongqing Science and Technology Bureau (cstc2020jscx-fyzx0227).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-188
Peer Review File: Available at http://dx.doi.org/10.21037/tp-20-188
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-188).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Castagnoli R, Votto M, Licari A, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr 2020;174:882-9. [Crossref] [PubMed]
- Wang Z, Zhou Q, Wang C, et al. Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann Transl Med 2020;8:620. [Crossref] [PubMed]
- Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp Pediatr 2020;10:537-40. [Crossref] [PubMed]
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771-8. [Crossref] [PubMed]
- Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607-8. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available online: https://emergency.cdc.gov/han/2020/han00432.asp
- Ge Y, Wang R, Chang H, et al. Epidemiology, clinical characteristics and discharge outcomes of children with COVID-19 based on eight case series studies and 10 case reports: A systematic review. Chinese Journal of Evidence-Based Pediatrics 2020;15:25-31.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Quality Appraisal of Case Series Studies Checklist. Edmonton (AB): Institute of Health Economics; 2014. Available online: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Deza Leon MP, Redzepi A, McGrath E, et al. COVID-19 Associated Pediatric Multi-System Inflammatory Syndrome. Journal of the Pediatric Infectious Diseases Society 2020;9:407-8. [Crossref] [PubMed]
- Dolinger MT, Person H, Smith R, et al. Pediatric Crohn Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated With Infliximab. J Pediatr Gastroenterol Nutr 2020;71:153-5. [Crossref] [PubMed]
- Waltuch T, Gill P, Zinns LE, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med 2020;38:2246.e3-6. [Crossref] [PubMed]
- Miller J, Cantor A, Zachariah P, et al. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology 2020;159:1571-1574.e2. [Crossref] [PubMed]
- Chiotos K, Bassiri H, Behrens EM, et al. Multisystem Inflammatory Syndrome in Children during the COVID-19 pandemic: a case series. Journal of the Pediatric Infectious Diseases Society 2020;9:393-8. [Crossref] [PubMed]
- Cheung EW, Zachariah P, Gorelik M, et al. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 2020;324:294-6. [Crossref] [PubMed]
- Rivera-Figueroa EI, Santos R, Simpson S, et al. Incomplete Kawasaki Disease in a Child with Covid-19. Indian Pediatr 2020;57:680-1. [Crossref] [PubMed]
- Greene AG, Saleh M, Roseman E, et al. Toxic shock-like syndrome and COVID-19: A case report of multisystem inflammatory syndrome in children (MIS-C). Am J Emerg Med 2020;38:2492.e5-6. [Crossref]
- Acharyya BC, Acharyya S, Das D. Novel Coronavirus Mimicking Kawasaki Disease in an Infant. Indian Pediatr 2020;57:753-4. [Crossref] [PubMed]
- Rauf A, Vijayan A, John ST, et al. Multisystem Inflammatory Syndrome with Features of Atypical Kawasaki Disease during COVID-19 Pandemic. Indian J Pediatr 2020;87:745-7. [Crossref] [PubMed]
- Chiu JS, Lahoud-Rahme M, Schaffer D, et al. Kawasaki Disease Features and Myocarditis in a Patient with COVID-19. Pediatr Cardiol 2020;41:1526-8. [Crossref] [PubMed]
- Ramcharan T, Nolan O, Lai CY, et al. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol 2020;41:1391-401. [Crossref] [PubMed]
- Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020;324:259-69. [Crossref] [PubMed]
- Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020;79:999-1006. [Crossref] [PubMed]
- Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020;142:429-36. [Crossref] [PubMed]
- Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 2020;10:69. [Crossref] [PubMed]
- Labé P, Ly A, Sin C, et al. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol 2020;34:e539-41. [Crossref] [PubMed]
- Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094. [Crossref] [PubMed]
- Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19: Case Series. Radiology 2020;297:E283-8. [Crossref] [PubMed]
- Balasubramanian S, Nagendran TM, Ramachandran B, et al. Hyper-inflammatory Syndrome in a Child With COVID-19 Treated Successfully With Intravenous Immunoglobulin and Tocilizumab. Indian Pediatr 2020;57:681-3. [Crossref] [PubMed]
- Yozgat CY, Uzuner S, Bursal Duramaz B, et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol Ther 2020;33:e13770. [PubMed]
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2950-73. [Crossref] [PubMed]
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020;382:e38. [Crossref] [PubMed]
- Zaim S, Chong JH, Sankaranarayanan V, et al. COVID-19 and Multiorgan Response. Curr Probl Cardiol 2020;45:100618. [Crossref] [PubMed]
- Liu E, Smyth RL, Luo Z, et al. Rapid advice guidelines for management of children with COVID-19. Ann Transl Med 2020;8:617. [Crossref] [PubMed]
- Zhang J, Yang Y, Yang N, et al. Effectiveness of intravenous immunoglobulin for children with severe COVID-19: a rapid review. Ann Transl Med 2020;8:625. [Crossref] [PubMed]
- Lu S, Zhou Q, Huang L, et al. Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis. Ann Transl Med 2020;8:627. [Crossref] [PubMed]
- Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, et al. A Retrospective Controlled Cohort Study of the Impact of Glucocorticoid Treatment in SARS-CoV-2 Infection Mortality. Antimicrob Agents Chemother 2020;64:e01168-20. [Crossref] [PubMed]
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 2020;395:1517-20. [Crossref] [PubMed]
- Belot A, Antona D, Renolleau S, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill 2020;25:2001010. [Crossref] [PubMed]
- World Health Organization. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- Royal College of Paediatrics and Child Health, editor. Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. UK: Royal College of Paediatrics and Child Health, 2020.



