Pneumatically-driven external pressure applicator to augment Fontan hemodynamics: preliminary findings
Introduction
Advances in pharmacologic treatments and surgical optimization for single ventricle or Fontan patients have been limited in recent years, resulting in the need for alternative therapeutic options (1,2). Clinical studies indicate that 20-30% of Fontans fail by ten years. While most institutions report 95% postoperative survival rates, the 10-year survival rate is only 60% (3). A heart transplant for these patients is a treatment option, if they can survive the organ waiting period. There is a growing interest in mechanical assistance of the cavopulmonary circulation to improve venous return and lung perfusion (2). By introducing a pressure boost (2-5 mmHg) to the pulmonary circulation, the deleterious characteristics of the dysfunctional Fontan could be reversed to more normal physiologic levels.
Conventional mechanical cardiovascular assist devices require invasive, open-heart surgery and are designed specifically for adult patients suffering from congestive heart failure (CHF). The success of these devices in adults having CHF and biventricular circulation is well reported. Although mechanical circulatory support devices have been shown to mitigate the failing Fontan physiology prior to heart transplant, current research indicates device success as only a short-term bridge-to-transplant option (2). Additionally, these devices require an invasive procedure for implementation, and no clinically available device to-date has been designed to specifically augment the unique circulatory demands of a Fontan physiology. Although cavopulmonary assist devices are currently under developed, all require invasive-to-minimally invasive implantation and are far from reaching clinical use.
In an attempt to achieve the goals of mechanical circulatory assistance while reducing the surgical risk and cost to patients, we performed a feasibility study to investigate the benefits of externally applied pressure to the lower limbs as a preventative measure and long-term clinical treatment strategy for Fontan patients. We hypothesized that the application of circumferential pressure to the lower limbs will augment venous return. Enhancement to venous return will, in turn, improve blood flow to the pulmonary arteries and cardiac output.
Materials and methods
Compression technology
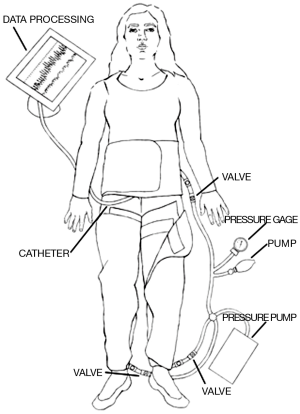
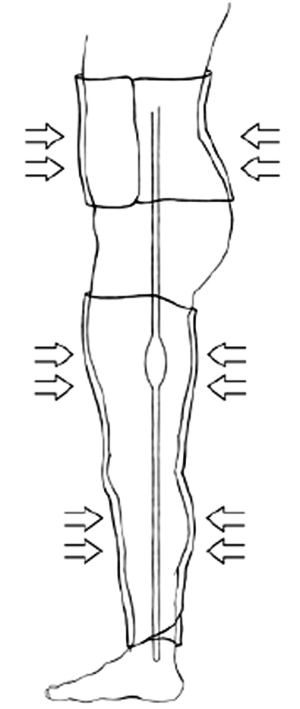
A set of commercial MAS trousers (David Clark Company, Worcester, MA) were retrofitted with an air-vacuum pump to control inflation and deflation in order to enable external pressure pulsation to the lower extremities. The pressure compartments were individual such that the abdominal and lower extremity sections were interchangeable. This provided allowance for some degree of personalized fit for the patients. The pressure application of the trousers was provided via two means of airflow arranged in series relative to the pressure garment. Bulk volume airflow to generate pressures accurate to within approximately ±5 mmHg originated from a standard air pump (Intertek Listed, Model: HB-505B). The air pump provided the coarse pressure adjustment to quickly elevate the pressure application to the patient. A hand pump, as found on most sphygmomanometers, was utilized for fine adjustments to within ±1 mmHg accuracy. A standard dial pressure gage was used to measure the applied pressure. Figure 1 illustrates the design of the retrofitted MAS-trousers.
Feasibility study
An approved feasibility study (n=2) of clinically-applied external compression by the institutional investigational review board (HM#11906) was performed using the MAS trousers. Neither of the participating subjects met any of the exclusion criteria. After obtaining informed consent, each patient was prepped for the scheduled cardiac catheterization. The subjects were placed in the supine position with the MAS trousers positioned around the lower extremities and midline torso, but not inflated. Standard ECG leads were appropriately located. Baseline measurements of blood pressure, respirations, aortic pressure, and heart rate were taken. Based on each patient’s diastolic pressure, we determined three separate pressure intervals to evaluate at levels of ±10 mmHg.
Following the baseline vital signs, the initial pressure level was applied. The MAS trousers were inflated, and a circumferential pressure was applied to the lower extremities similar to a large blood pressure cuff. This pressure was held for 40 seconds and then released for 20 seconds. The pressure was applied again. These intermittently applied pressures occurred for five minutes. At the conclusion of this first test period, the patient’s vital signs were reassessed, and the MAS trousers were deflated. The patient rested for five minutes. Then, the second pressure level was tested, which was slightly higher than the first interval, and after another rest period, the third and final pressure were applied. After five minutes of cycling compression, as previously described, a final set of vitals were obtained. Measurements of systemic blood pressure, pulmonary pressures, and pressure in the inferior vena cava (IVC) were simultaneously obtained during test intervals.
Results
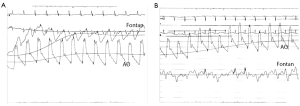
Mean baseline diastolic pressure for Patient 1 was 47 mmHg; thus, external pressure application was at 37, 47, and 57 mmHg. Patient 2 had a mean baseline diastolic pressure of 69 mmHg, and the external application was cycled at 59, 69, and 79 mmHg. Figure 2 illustrates sample pressure data for Patient 1 during upstroke of the applied pressure at the 2nd interval and, similarly, for Patient 2 at the 1st interval.
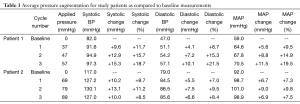
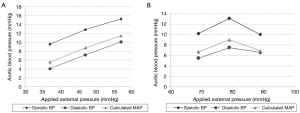
Both patients demonstrated augmentation in pressure levels (Figure 3). Table 1 outlines the average pressure augmentation for both patients as compared to the baseline measurements. Patient 1 developed measurable pressure augmentation during all three external pressure levels; the increase in pressure ranged from approximately 9-15 mmHg in systolic blood pressure, 4-10 mmHg in diastolic blood pressure, and 5-11 mmHg in mean arterial pressure (MAP). Average hemodynamic improvement in Patient 1 was found based on a measured pressure increase ranging from 10% during the first cycle to 20% during the third applied pressure level. Similarly, Patient 2 also developed detectable pressure increases during cycles of external pressure application by demonstrating systolic pressure augmentation of approximately 10-13 mmHg, diastolic increase of 5-7.5 mmHg and enhancement of MAP of 3-9 mmHg. Figure 4 displays the average aortic pressure augmentation as function of applied external pressure. Patient 1 demonstrated a linear trend in aortic pressure augmentation, while Patient 2 showed an unexpected decrease in the augmentation at the higher externally applied pressure.
Full table
Discussion
The absence of a pulmonary ventricle in Fontan patients places limitations on the amount of energy available to drive blood through the pulmonary vascular bed. Surgical modifications to the original Fontan procedure, coupled with better management strategies, have improved patients outcomes reducing post-operative mortality to the level of simpler defect repairs (4,5). However, Fontan patients are subjected to numerous long-term complications, such as thromboemboli, arrhythmias, ventricular dysfunction, pulmonary arteriovenous malformations (PAVMs), and protein losing enteropathy. There are also limited treatment options for failing Fontans. The prospect of pulsation technology for Fontans depends upon its ability to intermittently and positively “energize” the cavopulmonary circulation. The effect of this pulsation must produce advantageous biofluid dynamic conditions to promote forward blood blow, streamlined flow characteristics, minimal irregular flow patterns, and no flow statsis—all of which pervasively exist in the cavopulmonary junction and connecting vasculature.
Due to the demonstration of success in adult CHF patients and a study by Ma et al. (6) in 2002 of externally applied pulsation postoperatively on Fontan patients, we expected that similar technology (i.e., retrofitted MAS trouser configuration) would have a positive impact on older Fontan patients. We based our assumptions on the symptomatic and physiological similarities between these two categorical patients. Their pathological conditions arise from the common source of poor single ventricle myocardial function. Therefore, we executed this feasibility study using commercially available and retrofitted MAS trousers as new technology for patients having Fontan dysfunction.
Results from this study of two Fontan patients agree with literature in that MAS trousers successfully augmented venous return, cardiac output, and systemic blood pressure (7-10). Both patients demonstrated significant augmentation in pressure levels during external pressure application. Common trends for both patients included an increase in cardiac pressure during inflation holds and return to baseline during deflation holds. The cyclic inflation/deflation cycles are clearly visible for Patient 1; Patient 2 data reflected the cyclic inflation/deflation during the upper range of external pressure application.
In contrast to Patient 1, Patient 2 did not demonstrate a linear correlation between the external pressure application and resulting influence in aortic blood pressure. Patient 2 has a single ventricle with a classical Fontan, where the right atrium “lake” is preserved. During the clinical trial, the abdominal compartment section was not used, and hepatic vessel compliance during pressure application to the lower limbs may account for a portion of the pressure dissipation downstream in the expanding right atrium. Additionally during the catheterization, observations were made of patient respiratory rates and correlations to patient pulmonary arterial pressures. During deep sleep, it was noted that the patient’s respiratory phase variation had discernable effects on pulmonary arterial pressures.
A considerable limitation of this study is the lack of test subject population and of consistency in the type of Fontan configuration. Future clinical studies for continued development of the pressure garment will address these limitations by seeking multi-institutional trials. An increase in systemic vascular resistance (SVR) from the applied external pressure may also play a role in the pressure augmentation, as observed in this study. The test subjects were sedated for the cardiac catheterization procedure, which did not parallel actual usage of the device in the long-term, but provided insights in this acute setting. Furthermore, an increase in central venous pressure (CVP) for this patient population could have unintended consequences over a sustained time period; thus, we must consider the target patient population and level of ventricular dysfunction very carefully as we proceed with a larger, more comprehensive study. Cardiac and respiratory cycle synchronization to the externally applied compression will also be investigated. In Fontan patients, chest wall expansion during inhalation and subsequent depression during exhalation influence cardiac filling and ejection; in which case, such synchronization could maximize energy transfer to the systemic venous system. In addition, a square wave cycle was implemented for this study, and a range of ramping rates and durations were also investigated. Nevertheless, data from this feasibility study provided evidence that the external mechanical compression of lower vasculature increased blood pressure and cardiac output in both patients. Additional trials, including short-term outpatient testing, will be performed to statistically substantiate these conclusions and support the design and importance of this new and safe technology as a novel clinical management strategy for Fontan patients.
Acknowledgements
The authors wish to acknowledge the financial support for this work as provided by the U.S. Department of Education GAANN Interdisciplinary Graduate Engineering Education and Research (I-GEEAR) fellowship awards (S.S. Bhavsar and S.G. Chopski). The technology was developed at the Virginia Commonwealth University, Richmond, Virginia. The clinical study was also performed at the Virginia Commonwealth University. The prototype was constructed with assistance by 3-D Design and Manufacturing LLC, Powhatan, Virginia.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- de Leval MR, Deanfield JE. Four decades of Fontan palliation. Nat Rev Cardiol 2010;7:520-7. [PubMed]
- Throckmorton AL, Chopski SG. Pediatric circulatory support: current strategies and future directions. Biventricular and univentricular mechanical assistance. ASAIO J 2008;54:491-7. [PubMed]
- Lamour JM, Kanter KR, Naftel DC, et al. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol 2009;54:160-5. [PubMed]
- Piran S, Veldtman G, Siu S, et al. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002;105:1189-94. [PubMed]
- Rychik J, Spray TL. Strategies to treat protein-losing enteropathy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5:3-11. [PubMed]
- Ma W, Lemler MS, Nikaidoh H, et al. Usefulness of external counterpulsation early postoperatively after the Fontan procedure in children. Am J Cardiol 2002;90:1029-31. [PubMed]
- Loh PH, Cleland JG, Louis AA, et al. Enhanced external counterpulsation in the treatment of chronic refractory angina: a long-term follow-up outcome from the International Enhanced External Counterpulsation Patient Registry. Clin Cardiol 2008;31:159-64. [PubMed]
- Ng AV, Hanson P, Aaron EA, et al. Cardiovascular responses to military antishock trouser inflation during standing arm exercise. J Appl Physiol (1985) 1987;63:1224-9. [PubMed]
- Michaels AD, Accad M, Ports TA, et al. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation 2002;106:1237-42. [PubMed]
- Kemeny A, Geddes LA. Retrospectroscope. Military antishock trousers. IEEE Eng Med Biol Mag 2005;24:80, 91.