Chromosomal instability in first trimester miscarriage: a common cause of pregnancy loss?
IntroductionOther Section
Genetic instability results from phenotypic changes and mutations which are the consequence of metabolism and the environment. It is the instigator of both evolution and cancer. Instability may be observed as aneuploidy (or more rarely, polyploidy), chromosomal instability (CIN) or jumping translocations (JTs) (1).
Aneuploidy is the state where a cell contains an abnormal number of chromosomes divergent from normal diploid (2). Segmental aneuploidy occurs when unbalanced alterations result from DNA breakage and repair; giving rise to deletions, duplications, and unbalanced translocations (1). Aneuploidy does not necessarily predispose to cancer.
CIN in contrast, relates to a state of dynamic chromosomal flux of gains, losses and structural rearrangements. Aneuploidy can be the result of CIN but cannot be considered a substitute (3). CIN is one of the hallmarks of cancer cells and plays an important role in tumorigenesis (4). Although CIN may or may not promote tumor development, it is recognised as correlating to patient prognosis. Extreme CIN conflicts even with cell viability (3). In some instances, CIN can be induced or accelerated. For example, radiation was used to induce a translocation between chromosome 2 and 17 leading to mutations of alleles at the break sites (5).
JTs are a rare chromosomal event. They involve one specific breakpoint on a chromosome, termed the donor, that is then involved in structural rearrangements with multiple recipient chromosomes. JTs were originally described in constitutional cases (6-8). More recently they have been mainly reported in haematological malignancies and correlated with poor prognosis (9,10). There have been rare reports in constitutional studies in the live population. In a review by Reddy [2010] (11), 49 cases were identified in the literature, in addition to the twin pregnancy presented in the paper. JTs may be simple or complex. For example, a child with four different cell lines involving donor 15q material was reported (12). Other cases describe a meiotic transfer where the same donor exhibits different recipient chromosomes from parent to child, each being apparently balanced rearrangements (13,14).
There have been a few reports of prenatally identified JTs. Donor chromosomes include 22q11.2 (15,16), 18p11.1 (17) and 21p10 (18,19). Early studies suggested the involvement of mainly acrocentric chromosomes with the breakpoints containing repetitive DNA sequences in telomeric, centromeric or heterochromatic regions. Although the recipient chromosomes were random, there seems to be a preference for telomeric sequences (7,20,21). It has been suggested that JTs are formed by illegitimate recombination between interstitial telomeric sequences and telomere repeat sequences (22). The review by Reddy [2010] (11) examined chromosome and breakpoint involvement across all the publications. Although the majority of breakpoints were in heterochromatin or regions of known repeats (such as those involving Prader Willi Syndrome on 15q or the DiGeorge/velo-cardio-facial region on 22q11.2), approximately 27% donor sites and 5% recipient sites were in interstitial euchromatic regions.
First trimester miscarriage without underlying medical conditions is most commonly caused by chromosomal abnormalities reported to occur in 50% or more of cases (23). This differs markedly from the live born population where a review across 11 countries in Europe yields a chromosome abnormality rate of less than 0.5% (24). These chromosomal changes in early losses include both numerical abnormalities and structural alterations that result in gain and/or loss of genetic information, as few of these chromosomal imbalances are viable past the first trimester. Even the chromosomal abnormalities that are viable, such as monosomy X and trisomy 21, are often lost during the first twelve weeks after conception.
JTs were first described in miscarriages by Jacobs et al. [1974] (13), who reported a mother (the proband) with a maternally inherited apparently balanced whole arm translocation of 14q10 and 6p10. This translocation was also present in the sister and one aunt. The aunt had given birth to a son with the same chromosome 14 translocated to 15q10. The proband had a history of two early miscarriages and subsequently miscarried at 21 weeks. None of these losses were examined chromosomally. Tomkins [1981] (14) reported a family with the index miscarriage sample exhibiting an 11p11 translocated to 15p12 from a maternal apparently balanced reciprocal translocation between 11p11 and 22q12. Both of these cases were from familial alterations.
More recently, two cases of JTs were published by Levy et al. in 2000 (25), and two others by Lee et al., in 2010 (26). These four cases were all de novo, and may be better described as CIN, or re-termed jumping alterations, as the rearrangements involved not just translocations, but inversions, deletions and whole chromosome loss.
We present an observational study of 12 retrospective cases identified during routine cytogenetic analysis of first trimester miscarriage samples. All samples exhibited CIN. In these examples, we observed numerous chromosomal alterations in different cell populations. Some may be considered to be JT, where a single donor site was observed with different recipients. Others involved more than one site on the “donor” chromosome. One reported miscarriage involved multiple aneuploidy. All alterations resulted in partial trisomies and monosomies which predisposed the pregnancy to chromosomal imbalance and subsequent demise. To our knowledge this is the first report of such a large cohort in a single study population.
MethodsOther Section
The 12 patients reported herein were identified during routine clinical cytogenetic evaluation of failed pregnancies. Standard laboratory protocols for tissue culture, harvesting and analysis were in accordance with that published in Hardy et al. in 2016 (27). As this was a retrospective study obtained from laboratory records, no ethics approval was necessary. All patients have been de-identified. All material and data are stored according to NHMRC regulations.
ResultsOther Section
We report 12 examples of chromosome instability seen in the fetal material of spontaneous first trimester miscarriages (Tables 1,2). In these samples related chromosomal alterations were observed in different cell clones. Some may be considered to be JT, where a single donor site was observed with different recipients. Others involved more than one site on the “donor” chromosome.
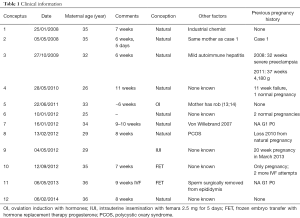
Full table

Full table
All pregnancies miscarried within the first trimester (6–11 weeks). Eight patients were attending a reproductive clinic, although only four mothers required intervention to achieve pregnancy; two involving embryo transfer, and the other two conceived with hormone assistance. All other conceptions were spontaneous pregnancies. Maternal age varied from 25 to 36 years of age at the time of miscarriage. One patient worked as an industrial chemist and exhibited two pregnancy failures with CIN (numbers 1 and 2). There were no other patients with any identified risks from environmental or mutagenic exposure.
The past pregnancy history of the patients varied from no previous pregnancy, through miscarriage and normal pregnancy, to later pregnancies with medical complications, and no further pregnancies even with IVF attempts (Table 1).
One conception involved multiple aneuploidy variations. All conceptions demonstrated partial trisomies and/or monosomies. Donor chromosomes included 2, 5, 8, 11, 12, 14, 16, and 22. Two conceptions exhibited more than one breakpoint in the donor chromosome: chromosome 5 (conception 6) (Figure 1) and chromosome 22 (conception 5). Another conception exhibited a common breakpoint on chromosome 11 and two different breakpoints on the long arm of chromosome 9 (conception 7). The most complex conception (number 12) exhibited multiple chromosomal aneuploidy, where some common errors were present in two clones, although extensive cell to cell variability was observed. Of the twelve pregnancies, three sets of parents did not have cytogenetic studies done in our facility. Conceptions 3, 6 and 9 (Figure 2) exhibited a normal cell line, suggesting that the errors were post-zygotic. Conception 12 exhibited numerical alterations, which would not be constitutional in a parent.
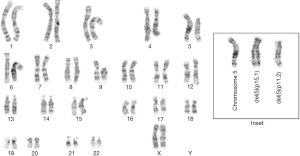
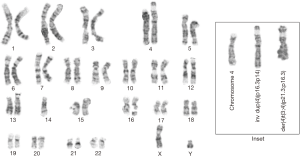
Cytogenetic observations
The karyotypes are presented in Table 2.
Conceptus 1
Two cell lines with a common breakpoint on 8p11.2. Half the cells were deleted for the terminal region of the short arm, while the other half exhibited an unbalanced derivative with additional 1q24 to the telomere.
Conceptus 2
Two cell lines with a common breakpoint at 17q25. The majority of cells had unidentified additional material terminal to this breakpoint. A minor clone exhibited a derivative unbalanced translocation with a breakpoint on11q13. The mother of this conception is the same as for Conceptus 1.
Conceptus 3
This conception exhibited five different clones. There was a common breakpoint on chromosome 12 at p13.1 in four populations. All four clones were monosomic for the terminal region of the short arm of chromosome 12. Partial trisomy 12, 9, unknown origin (or most likely 17) was present in the respective clones. The conception also exhibited a normal karyotype in approximately one third of cells.
Conceptus 4
Chromosome 2 exhibited a breakpoint at p15. Clone 1 partial monosomy 2p; clone 2 partial trisomy 9q.
Conceptus 5
This conception inherited a Robertsonian 13;14 translocation from the mother. Five additional cell lines were identified, each with different alterations to chromosome 22. Two different breakpoints occurred; one at q11.2, and the other at q13. The final clone was monosomic for chromosome 22.
Conceptus 6
Two different breakpoints on 5p resulting in deletions of the short arm terminal to the breakpoint.
Conceptus 7
This conception exhibited two different unbalanced translocations between chromosomes 9 and 11. The breakpoint on chromosome 11 was constant at q23.3, while two different breakpoints on chromosome 9 were identified: one at q13 and the other at q22.3.
Conceptus 8
Two different alterations to chromosome 16 at p13.3 were observed. Material distal to the breakpoint was not identified in the major clone, while trisomy 17q was observed in clone 2.
Conceptus 9
This conception was mosaic for normal male and two different rearrangements at 4p16.3. One cell line was duplicated for 4p16.3 to 4p14. The second cell line contained an unbalanced derivative from a 4p16.3;3p21.3 translocation.
Conceptus 10
Four tetraploid cell lines were observed; one normal, two with different alterations at 14q11.2 and the final clone with loss of 14.
Conceptus 11
The majority of cells in this conception exhibited a derivative chromosome 8 from an 8;8 translocation, with breakpoints at 8p21.3 and 8q13. The second cell line was deleted at 8p21.3, resulting in monosomy of the distal region.
Conceptus 12
The cytogenetic result was highly complex. Two different clones with common changes were identified amongst the complexity of individual cells. Clone 1 exhibited additional copies of chromosomes 2, 8, 12 and a marker. Clone 2 contained two additional copies of 1, an additional deleted 1q, additional copies of 2, 5, del12p11, and variable copies of a marker chromosome.
DiscussionOther Section
The 12 conceptions in this report are not consistent with many of the normal observations of JT. Conceptions 5 and 6 demonstrated an unstable chromosome, rather than just one breakpoint. Conceptus 12 predominantly exhibited numerical alterations, in addition to structural errors which resulted in extensive genetic imbalance.
The observation of JTs in miscarriage has been rarely reported. The first and second cases in the literature arose from meiotic alteration from a parental translocation (13,14). Four more cases in two manuscripts were presented where several cell lines were observed with donor chromosomes and different recipients; all of which were de novo events (25,26). An additional case of a slightly later pregnancy loss described a twin pregnancy with differing cytogenetic results involving the same donor chromosome (11). Many JTs breakpoints reported in the published literature are centromeric or telomeric. In contrast, this current data set presents multiple instances where they are not (numbers 3–9 and 11). In addition, this set of conceptions not only involves translocations, it also identifies different rearrangements within the same donor chromosome: inversions, complete loss of the chromosome, deletion of the chromosome distal to the breakpoint, and more than one alteration to the same chromosome arm (deletions with varied breakpoints, pseudo-isodicentric). It may be more correct to identify these events as CIN rather than purely JT. Conceptus 12 differs in that it appears to exhibit extensive variability, both in numerical and structural alterations.
Furthermore, though two instances of acrocentric donor chromosomes were identified (numbers 5 and 10), in most of the current data set the donor chromosome was not an acrocentric. The breakpoint on each acrocentric was on the q arm and not centromeric, although the breakpoint on chromosome 22 did occur at q11.2, the region involved in many genetic conditions (velocardiofacial/DiGeorge). It may therefore be suggested that the molecular mechanisms of these rearrangements may differ from those observed in haematological malignancies, in keeping with the conclusions of Reddy [2010] (11).
This is the first time such a large number of JT’s and/or CIN has been reported within a single study population of first trimester miscarriages. Indeed, never before has this many examples of instability been reported in one population of first trimester losses. They were observed in a population of 2,258 successfully karyotyped samples, thus representing 0.5%. The total study population comprised 2,445 samples with a culture success rate of 92.4% and an abnormality rate of 74.5%.
The above reported percentage is higher than:
- Trisomies of chromosomes 3, 5, 6, 11, 12, 17, 19 and X;
- XXY;
- Autosomal monosomies (27).
In our population, structural alterations were observed in 2.9% of first trimester miscarriages (27). Other studies range from 1.2% (28) to 4.4% (29).
In conclusion, given the high rate of occurrence, the phenomena should be recognised as a significant contributor to genetic imbalance causing fetal demise. Therefore, it is important to integrate such understanding into testing protocols.
Parents experiencing CIN in miscarriage are able to have normal pregnancies, but in many cases, have other reproductive problems. Although preliminary (with only one patient), there is a chance that CIN may occur in future pregnancies. Environmental factors may be involved in causing CIN in miscarriage; our data suggests that it is unlikely to be the only cause.
Further understanding of the mechanisms resulting in genetic instability may give us insight into the reason for fetal demise in these cases. By the use of single cell molecular tools, it may be possible in the future to uncover the mechanism or better understand the result of CIN in early development.
AcknowledgementsOther Section
Many thanks to Professor Terry Hassold for careful reading of the manuscript and helpful comments during preparation of the document.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As this was a retrospective study obtained from laboratory records, no ethics approval was necessary. All patients have been de-identified. All material and data are stored according to NHMRC regulations.
ReferencesOther Section
- Geigl JB, Obenauf AC, Schwarzbraun T, et al. Defining “chromosomal instability’. Trends Genet 2008;24:64-9. [Crossref] [PubMed]
- McGowan-Jordan J, Simons A, Schmid M. editors. ISCN 2016: An international system for human cytogenomic nomenclature. Kargar Basel 2016:90.
- Anderhub SJ, Krämer A, Maier B. Centrosome amplification in tumorigenesis. Cancer Letters 2012;322:8-17. [Crossref] [PubMed]
- Michor F, Iwasa Y, Vogelstein B, et al. Can chromosomal instability initiate tumorigenesis? Semin Cancer Biol 2005;15:43-9. [Crossref] [PubMed]
- Woychik RP, Generoso WM, Russell LB, et al. Molecular and genetic characterization of a radiation-induced structural rearrangement in mouse chromosome 2 causing mutations at the limb deformity and agouti loci. Proc Natl Acad Sci 1990;87:2588-92. [Crossref] [PubMed]
- Gimelli G, Porro E, Santi F, et al. “Jumping” satellites in three generations: a warning for paternity test and prenatal diagnosis. Hum Genet 1976;34:315-8. [Crossref] [PubMed]
- Lefort G, Blanchet P, Chaze AM, et al. Cytogenetic and molecular study of a jumping translocation in a baby with Dandy-Walker malformation. J Med Genet 2001;38:67-73. [Crossref] [PubMed]
- Lejeune J, Maunoury C, Prieur M, et al. Translocation sauteuse (5p;15q), (8q;15q), (12q;15q). Annals of Genetics 1979;22:210-3.
- Reis MD, Dube ID, Pinkerton PH, et al. “Jumping” translocations involving band 3q13.3 in a case of acute monocytic leukemia. Cancer Genet Cytogenet 1991;51:189-94. [Crossref] [PubMed]
- Ben-Neriah S, Abramov A, Lerer I, et al. “Jumping translocation” in a 17-month-old child with mixed-lineage leukemia. Cancer Genet Cytogenet 1991;56:223-9. [Crossref] [PubMed]
- Reddy KS. The conundrum of a jumping translocation (JT) in CVS from twins and review of JTs. Am J Med Genet Part A 2010;152A:2924-36. [Crossref] [PubMed]
- Jewett T, Marnane D, Stewart W, et al. Jumping translocation with partial duplications and triplications of chromosomes 7 and 15. Clin Genet 1998;53:415-20. [Crossref] [PubMed]
- Jacobs PA, Buckton KE, Christie S, et al. A family with two translocations and a polymorphism involving chromosome 14. J Med Genet 1974;11:65-8. [Crossref] [PubMed]
- Tomkins DJ. Unstable familial translocations: A t(11;22)mat inherited as a t(11;15). Am J Hum Genet 1981;33:745-51. [PubMed]
- Aslan H, Karaman B, Yildirim G, et al. Prenatal diagnosis of jumping translocation involving chromosome 22 with ultrasonographic findings. Prenat Diagn 2005;25:1024-7. [Crossref] [PubMed]
- Blumberg B, Ward B, Letterer D. Jumping translocations and their phenotypic consequences. Proc Greenwood Genet Cent 1990;10:54.
- Edwards S, Waters JJ. Prenatal diagnosis of monosomy 18p involving a jumping translocation. Prenat Diagn 2008;28:764-6. [Crossref] [PubMed]
- Annable K, Donnenfeld AE, Fischer RL, et al. Prenatal diagnosis of a jumping translocation. Prenat Diagn 2008;28:767-9. [Crossref] [PubMed]
- Hulley BJ, Bleigh CB, McAdoo SL, et al. “Jumping translocation” involving 21p in amniocytes. J Assoc Genet Technol 2003;29:91. [PubMed]
- Park VM, Gustashaw KM, Wathen TM. The presence of interstitial telomeric sequences in constitutional chromosome abnormalities. Am J Hum Genet 1992;50:914-23. [PubMed]
- Vermeesch JR, Petit P, Speleman F, et al. Interstitial telomeric sequences at the junction site of a jumping translocation. Hum Genet 1997;99:735-7. [Crossref] [PubMed]
- Jamet D, Marzin Y, Douet-Guilbert N, et al. Jumping translocations in multiple myeloma. Cancer Genet Cytogenet 2005;161:159-63. [Crossref] [PubMed]
- Hardy K, Hardy PJ. 1st trimester miscarriage: four decades of study. Transl Pediatr 2015;4:189-200. [PubMed]
- Wellesley D, Dolk H, Boyd PA, et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet 2012;20:521-6. [Crossref] [PubMed]
- Levy B, Dunn TM, Hirschhorn K, et al. Jumping translocations in spontaneous abortions. Cytogenet Cell Genet 2000;88:25-9. [Crossref] [PubMed]
- Lee YW, Lee BY, Park JY, et al. Rarely Observed Jumping Translocation in Spontaneous Abortion. J Genet Med 2010;7:82-6. [Crossref]
- Hardy K, Hardy PJ, Jacobs PA, et al. Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. Am J Med Genet A 2016;170:2671-80. [Crossref] [PubMed]
- Byrne J, Warburton D, Kline J, et al. Morphology of early fetal deaths and their chromosomal characteristics. Teratology 1985;32:297-315. [Crossref] [PubMed]
- Eiben B, Borgmann S, Schubbe I, et al. A cytogenetic study directly from chorionic villi of 140 spontaneous abortions. Hum Genet 1987;77:137-41. [Crossref] [PubMed]


