
Comparison of diagnostic criteria for sepsis-associated acute kidney injury in the pediatric intensive care unit: a retrospective cohort study
Highlight box
Key findings
• pRIFLE (Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease) is more sensitive in early detection of sepsis-associated acute kidney injury (SA-AKI).
What is known and what is new?
• The diagnostic criteria for AKI are constantly being revised, but there is still no unique accepted definition.
• SA-AKI diagnostic consistency by three classification criteria is similar. pRIFLE is more sensitive in early detection. pROCK (Pediatric Reference Change Value Optimized for AKI) has higher specificity.
What is the implication, and what should change now?
• Clinicians should improve the understanding of SA-AKI according to different medical history characteristics and choose the appropriate scoring method to carry out early assessment of AKI.
Introduction
Acute kidney injury (AKI), characterized by abrupt deterioration in kidney function, manifests as an increase in serum creatinine level or reduced urine output (UO) and is a common complication of critically ill patients. Two studies involving 128 pediatric intensive care units (PICUs) in 26 countries showed that more than half of AKI cases were considered to be closely related to sepsis and were an important cause of death in children (1,2). Sepsis-associated AKI (SA-AKI) has been associated with poor outcomes, including increased length of stay in the PICU, higher mortality, and higher healthcare costs.
However, due to the difficulty in collecting urine in children, the use of diuretics, serum creatinine in SA-AKI children not always accurately reflecting the glomerular filtration rate, and the time delay of laboratory results, relying solely on serum creatinine level or estimated glomerular filtration rate (eGFR) and changes in UO to diagnose SA-AKI may be challenging. Although our knowledge of AKI continues to increase, and the diagnostic criteria for AKI are constantly being revised, there is still no standard definition of this condition.
In 2007, the pRIFLE (Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease) criteria for diagnosing and staging AKI in children were proposed for the first time (3). The most widely used standard is the one established by the KDIGO (Kidney Disease Improving Global Outcomes) organization in 2012, which applies to both adults and children (4). In 2018, Xu et al. (5) proposed the pROCK (Pediatric Reference Change Value Optimized for AKI) standard for children based on the reference values of baseline creatinine in Chinese children. The wide variation in AKI definitions creates significant discrepancies across the results of studies, making comparison of epidemiological studies difficult.
Therefore, we aimed to assess the consistency and related clinical characteristics of diagnosing SA-AKI by comparing the incidence, risk factors, and prognostic differences of the SA-AKI of the pRIFLE, KDIGO, and pROCK standards. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-34/rc).
Methods
Study design
This retrospective cohort study included 62 patients treated for sepsis in the PICU, The First Affiliated Hospital of Anhui Medical University between January 1, 2019 and December 31, 2022. The inclusion criteria for patients were the following: (I) meeting the International Consensus Criteria for Pediatric Sepsis and Septic Shock (2024) (6) for sepsis, (II) an age ≥1 month and ≤18 years, (III) length of hospital stay of at least 48 hours, and (IV) creatinine testing performed at least twice during hospitalization. Meanwhile, the exclusion criteria were the following: (I) age ≥18 years, (II) a history of any kidney-related disease, (III) AKI caused by noninfectious factors, and (IV) end-stage multiple organ failure. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University (No. Quick-PJ-2023-16-10). All patient’s guardians consented to submission of this research to the journal and provided written informed consent.
Clinical information were collected, including general information, basic vital signs, basic medical history, laboratory outcomes [serum creatine (sCR), UO, and eGFR], mechanical ventilation support requirements, mechanical ventilation support duration, ICU hospitalization status, various critical illness scores [pediatric critical illness score (PCIS), pediatric risk of mortality (PRISM) III, multiple organ dysfunction score (MODS), acute physiological and chronic health evaluation (APACHE) II, and vasoactive infusion score (VIS)], major complications, and 28-day survival status.
Relevant definitions
SA-AKI refers to patients with no basic kidney disease and renal function impairment, resulting in abnormal kidney function and structure due to sepsis (6).
Blood creatinine was measured using the creatinine oxidase method, and eGFR was calculated using the Schwartz formula. We defined fluid overload (FO) as a cumulative fluid balance greater than 10% above baseline weight within 48 hours of sepsis recognition (7).
AKI classification
Due to the lack of UO per hour, the UO indicators in the pRIFLE and KDIGO standards were not used. AKI was diagnosed during the first 24 hours of PICU admission.
- The diagnostic staging of AKI in the 2007 pRIFLE standard is an eGFR decrease of ≥25% within 7 days (3). AKI stage 1 is an eGFR decrease of ≥25% and <50%, AKI stage 2 is an eGFR decrease of ≥50% and <75%, and AKI stage 3 is an eGFR decrease of ≥75%.
- The KDIGO definition of AKI requires meeting one of the two following criteria: increase in sCR level to ≥1.5 times the baseline within the previous 7 days or a ≥0.3 mg/dL increase in sCR occurring within 48 h (4). AKI is divided into three stages according to the degree of increment in sCR level: AKI stage 1 is a serum creatinine increase of ≥26.5 µmol/L or a serum creatinine increase value/baseline value of ≥50% and <100%, AKI stage 2 is a sCR level increase value/baseline value of ≥100% and <200%, and AKI stage 3 is a sCR level increase value/baseline value of ≥200%.
- AKI diagnosis and staging according to the 2018 pROCK standard is a blood creatinine increase of ≥20 µmol/L and a 30% increase from the baseline value within 7 days. According to the ratio of the absolute value of blood creatinine increase to baseline value, AKI is divided into three stages: AKI stage 1 is a blood creatinine increase value of ≥20 and <40 µmol/L and an sCR increase value/baseline value of ≥30% and <60%, AKI stage 2 is a blood creatinine increase value of ≥40 and <80 µmol/L and an increase in sCR value/baseline value of ≥60% and <120%, and AKI stage 3 is an increase in blood creatinine value of ≥80 µmol/L and an increase in sCR value/baseline value of ≥120% (Table 1).
Table 1
| Classification | Content |
|---|---|
| pRIFLE (3) | |
| AKI 1 | Decrease in eGFR ≥25%, and <50% |
| AKI 2 | Decrease in eGFR ≥50%, and <75% |
| AKI 3 | Decrease in eGFR ≥75% |
| KDIGO (4) | |
| AKI 1 | Increase in sCR ≥26.5 μmol/L, or increase in sCR/baseline ≥50%, and <100% |
| AKI 2 | Increase in sCR/baseline ≥100%, and <200% |
| AKI 3 | Increase in sCR/baseline ≥200% |
| pROCK (5) | |
| AKI 1 | Increase in sCR ≥20 μmol/L, <40 μmol/L, and increase in sCR/baseline ≥30%, and <60% |
| AKI 2 | Increase in sCR ≥40 μmol/L, <80 μmol/L, and increase in sCR/baseline ≥60%, and <120% |
| AKI 3 | Increase in sCR ≥80 μmol/L, and increase in sCR/baseline ≥120% |
pRIFLE, Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease; KDIGO, Kidney Disease Improving Global Outcomes; pROCK, Pediatric Reference Change Value Optimized for AKI; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; sCR, serum creatine.
Patients in this study were divided into two groups: AKI (−) group and AKI (+) group.
Baseline sCR value
For children who have never undergone blood creatinine testing before, if creatinine results are within the normal range after admission, then these creatinine results are used as the baseline value; for children whose first blood creatinine level after admission is elevated, the upper limit of normal sCR level in healthy children of this age group is used as the baseline value (8,9).
Outcomes
Firstly, the differences among the pRIFLE, pROCK and KDIGO classifications in the diagnosis and staging of AKI were examined. Secondly, the risk and predictive factors of AKI in patients with septic shock were analyzed.
Statistical analyses
All statistical analyses were performed using SPSS 22.0 (IBM Corp., NY, USA) and JMP 15.0 software. Categorical variables are expressed as percentages and were analyzed with the chi-squared and Fisher exact tests. Continuous variables with a Gaussian distribution are expressed as ; while those with a nonnormal distribution are expressed as the median with interquartile range (IQR). For normally distributed measurement data, a t-test was used to compare two groups, while for count data, a chi-squared test was used. Cohen’s kappa analysis was used to evaluate the diagnostic consistency of different AKI standards. κ≤0.4 was defined as poor consistency, and κ>0.6 was defined as good consistency. Univariate regression analysis was used to model the factors affecting AKI and mortality, and then a multivariate regression analysis model was established based on the univariate test model. The receiver operation characteristic (ROC) curve was used to evaluate the predictive value of relevant parameters for SA-AKI. Survival analysis was performed using the Kaplan-Meier method. The significance level used for the tests was P<0.05. The sample size was small and no outlier judgment was performed.
Results
General information of children with sepsis
Within the same period, 121 children with sepsis were admitted to the PICU, including 67 cases of severe sepsis. Two children died within 24 hours of hospitalization, two were excluded due to combined hemopoietic system malignant tumor, and one was excluded due to congenital kidney disease. A total of 62 children who met the inclusion criteria were recruited, including 42 males (67.74%) and 20 females (32.26%), with a median age of 23 months (1–175 months). The infection source was the blood stream in 11 cases (17.74%), the central nervous system in 10 cases (16.12%), the abdomen in 9 cases (14.52%), the pulmonary system in 9 cases (14.52%), the urinary tract in 9 cases (14.52%), the skin in 6 cases (9.68%), and an unknown infection source in 8 cases (12.90%). There were 29 patients with septic shock.
Incidence of AKI under different diagnostic criteria
Under the pRIFLE, KDIGO, and pROCK diagnostic criteria, about 53.2% (33/62) of children were diagnosed with AKI, and the incidence of AKI diagnosed by the three criteria sets was 74.2% (46/62), 67.7% (42/62), and 56.5% (35/62), respectively, with pRIFLE having higher incidence of AKI. According to paired chi-squared test, there was no significant difference between the pRIFLE standard and KDIGO standard (χ2=37.316; P=0.22), but there was a significant difference between the pRIFLE standard and the pROCK standard (χ2=20.558; P=0.002) and between the KDIGO standard and pROCK standard (χ2=35.850; P=0.008). The incidence of AKI staging in children with sepsis diagnosed by the different standards is shown in Table 2, with AKI stage 1 being the most common, accounting for 41.9% (26/62), 25.8% (16/62), and 19.4% (12/62), according to the pRIFLE, KDIGO, and pROCK standards, respectively.
Table 2
| Classification | pRIFLE, n (%) | KDIGO, n (%) | pROCK, n (%) |
|---|---|---|---|
| AKI (–) | 16 (25.8) | 20 (32.3) | 27 (43.5) |
| AKI (+) | |||
| Stage 1 | 26 (41.9)b | 16 (25.8)b,c | 12 (19.4)a |
| Stage 2 | 13 (21.0)b | 16 (25.8)b,c | 13 (21.0)a |
| Stage 3 | 7 (11.3)b | 10 (16.1)b,c | 10 (16.1)a |
a, compared with the incidence of AKI according to pRIFLE criteria (P<0.05); b, compared with the incidence of AKI according to pROCK criteria (P<0.05). c, compared with the incidence of AKI according to pRIFLE criteria (P>0.05). AKI, acute kidney injury; pRIFLE, Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease; KDIGO, Kidney Disease Improving Global Outcomes; pROCK, Pediatric Reference Change Value Optimized for AKI.
Among the three standards used to diagnose AKI in children with sepsis, there was no statistical difference between children who met any diagnostic criteria and those who did not in terms of PICU admission time, age, sex, or use of nephrotoxic drugs (all P values >0.05); mean arterial pressure (MAP) and PCIS scores of patients with SA-AKI were lower than those without AKI, while the procalcitonin (PCT) level was higher in patients with SA-AKI than in those without AKI (all P values <0.01; Table 3). According to the Phoenix Sepsis Score (6), the MAP score was stratified by age, and the comparison results still indicated that most patients with SA-AKI were complicated with hemodynamic instability. In addition, under the pRIFLE criteria, the difference in albumin (ALB) levels between those with SA-AKI and those without non-AKI was significant. When KDIGO standard was used, patients with SA-AKI had higher lactic acid levels and were more likely to have combined shock, hypoalbuminemia, elevated urea levels, and a high blood purification treatment rate; moreover, they had a significantly higher MODS score, PRISM score, and APACHE (acute physiology and chronic health evaluation) score. When pROCK standard was used, the PCIS score and MAP of patients with SA-AKI were lower, and more of the patients with SA-AKI had septic shock and received blood purification treatment; moreover, various critical indices, including lactic acid level, PH value, ALB level, use of mechanical ventilation, and administration of vascular active drugs were significantly higher (Table 3).
Table 3
| Characteristics | pRIFLE | KDIGO | pROCK | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AKI (–) (n=16) | AKI (+) (n=46) | P | AKI (–) (n=20) | AKI (+) (n=42) | P | AKI (–) (n=27) | AKI (+) (n=35) | P | |||
| Age (months) | 37.0 (4.5, 133) | 23.0 (2.8, 80.3) | 0.21 | 13 (3, 121.5) | 25.5 (4.8, 97) | 0.74 | 13 (3, 120) | 25.5 (5, 102.5) | 0.57 | ||
| Male sex | 12 (28.6) | 30 (71.4) | 0.47 | 20 (47.6) | 22 (52.4) | 0.57 | 15 (35.7) | 27 (64.3) | 0.40 | ||
| PICU length of stay (days) | 9.5 (5, 15) | 7 (5, 10) | 0.17 | 7 (5, 13.75) | 7 (5, 10.3) | 0.65 | 6 (5, 12.5) | 7.5 (5, 11) | 0.63 | ||
| Mechanical ventilation | 8 (50.0) | 21 (45.7) | 0.78 | 8 (40) | 21 (50.0) | 0.59 | 9 (32.1) | 20 (58.8) | 0.04 | ||
| Dialysis | 3 (18.8) | 13 (28.3) | 0.53* | 2 (10.0) | 14 (33.3) | 0.07 | 2 (7.1) | 14 (41.2) | 0.003 | ||
| Nephrotoxin exposure | 5 (31.2) | 18 (39.1) | 0.77 | 6 (30.0) | 17 (40.5) | 0.58 | 8 (28.6) | 15 (44.1) | 0.29 | ||
| MAP (mmHg) | 59.06±10.55 | 51.33±11.72 | 0.02 | 58.75±10.01 | 50.74±11.88 | 0.01 | 58.00±9.69 | 49.47±12.2 | 0.004 | ||
| Vasopressor use | 3 (18.8) | 18 (39.1) | 0.22 | 2 (10.0) | 19 (45.2) | 0.009 | 4 (14.3) | 17 (50.0) | 0.006 | ||
| Fluid overload at 48 h | 5 (31.2) | 14 (30.4) | >0.99* | 5 (25.0) | 14 (33.3) | 0.57 | 5 (17.9) | 14 (41.2) | 0.06 | ||
| Septic shock | 4 (25.0) | 25 (54.3) | 0.08 | 4 (20) | 25 (59.5) | 0.006 | 7 (25) | 22 (64.7) | 0.002 | ||
| Relevant scale score | |||||||||||
| MODS | 8.5 (8, 9) | 9 (8, 12) | 0.14 | 8 (8, 9) | 10 (8, 12) | 0.005 | 8 (8, 9) | 10 (8, 12) | 0.003 | ||
| PRISM III score | 18.0 (14.0, 24) |
23.0 (15.75, 31.25) |
0.03 | 17.5 (13.25, 23.5) |
23.5 (16.75,32.5) |
0.04 | 17.5 (13.25, 23.75) |
24 (17, 34.25) |
0.02 | ||
| PCIS | 77.88±10.09 | 68.98±14.54 | 0.01 | 78.90±10.57 | 67.64±14.09 | 0.001 | 77.71±10.74 | 65.97±14.30 | <0.001 | ||
| APACHE II | 25.81±5.62 | 28.11±8.01 | 0.22 | 24.00±4.68 | 29.19±8.03 | 0.002 | 24.71±4.99 | 29.82±8.44 | 0.005 | ||
| Laboratory features | |||||||||||
| Lac (mmol/L) | 2.45 (1.95, 3.68) |
5.05 (2.18, 7.83) |
0.09 | 2.11 (1.81, 3.68) |
5.2 (2.5, 7.9) |
0.006 | 2.16 (1.73, 3.4) |
5.6 (3.35, 8.7) |
<0.001 | ||
| Platelets (×109/L) | 221.5 (129.75, 357.75) |
187 (75.75, 368.5) |
0.57 | 252.5 (146, 402.25) |
148 (73.75, 325.75) |
0.13 | 230.5 (146, 376) |
139.5 (69.5, 327.25) |
0.09 | ||
| Leukocyte (×109/L) | 11.07 (3.69, 19.12) |
10.4 (4.11, 20.73) |
0.75 | 8.83 (3.69, 16.03) |
14.09 (4.59, 22.12) |
0.24 | 8.83 (3.16, 16.8) |
15.22 (5.81, 22.12) |
0.14 | ||
| Na+ (mmol/L) | 133.9 (127.73, 137.33) |
135 (127, 138.85) |
0.59 | 133.9 (129.25, 137.98) |
135 (126.83, 138.85) |
0.98 | 135.15 (130.58, 138.9) |
133.05 (126.23, 138.5) |
0.30 | ||
| Arterial pH | 7.32 (7.21, 7.41) |
7.26 (7.16, 7.34) |
0.13 | 7.32 (7.22, 7.38) |
7.24 (7.13, 7.34) | 0.04 | 7.32 (7.24, 7.38) |
7.23 (7.1, 7.3) | 0.003 | ||
| CRP (mg/L) | 98.81 (31.3, 145.9) |
93.86 (63.16, 187.86) |
0.49 | 78.5 (31.3, 123.27) |
109.74 (64.63, 187.86) |
0.12 | 94.16 (64.08, 123.27) |
99.29 (59.88, 204.24) |
0.40 | ||
| PCT (ng/ml) | 2.65 (1.29, 13.24) |
22.76 (5.42, 58.9) |
<0.001 | 3.85 (1.61, 6.07) |
24.83 (11.89, 62.32) |
<0.001 | 5.64 (2.26,2 0.48) |
23.63 (7.06, 64.96) |
0.002 | ||
| Urea (mmol/L) | 5.25 (2.64, 13.98) |
5.8 (3.36, 12.12) |
0.46 | 3.53 (1.20, 8.04) |
6.12 (3.5, 15.28) |
0.04 | 4.78 (2.48, 8.04) |
7.5 (3.48, 16.96) |
0.06 | ||
| K+ (mmol/L) | 4.43 (4.11, 4.5) |
4.7 (3.78, 5.5) |
0.30 | 4.47 (4.11, 5.17) |
4.59 (3.78, 5.47) |
0.71 | 4.47 (3.86, 5.02) |
4.69 (3.78, 5.53) |
0.43 | ||
| ALB (g/L) | 33.65 (27.08, 39.23) |
30.2 (22.32, 35.45) |
0.05 | 33.4 (28.9, 38.93) |
30.15 (20.03, 35.45) |
0.03 | 33.95 (29.35, 38.7) |
28.95 (19.53, 33.1) |
0.001 | ||
| eGFR (mL/min/1.73 m2) | 169.75 (129.2, 249.22) |
125.23 (69.13, 144.28) |
0.02 | 146.9 (126.85, 207.1) |
122.9 (68.12, 191.62) |
0.06 | 138.7 (123, 213.14) |
118.4 (66.86, 187.1) |
0.02 | ||
| sCR (μmol/L) | 27.05 (21.9, 44.7) |
63.8 (43.86, 144.26) |
<0.001 | 27.55 (21.9, 38.3) |
72 (53.1, 153.8) |
<0.001 | 29.9 (24, 42.88) |
102.75 (61.75, 163.85) |
<0.001 | ||
| Clinical outcomes | |||||||||||
| Mortality at 28 d | 0 (0) | 7 (15.2) | 0.18 | 0 (0) | 7 (16.7) | 0.09* | 1 (3.6) | 6 (17.6) | 0.12* | ||
Data are presented as n (%), median (IQR) or mean ± SD. *, Fisher’s exact test. AKI, acute kidney injury; pRIFLE, Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease; KDIGO, Kidney Disease Improving Global Outcomes; pROCK, Pediatric Reference Change Value Optimized for AKI; PICU, pediatric intensive care unit; MAP, mean arterial pressure; MODS, multiple organ dysfunction score; PRISM, pediatric risk of mortality; PCIS, pediatric critical illness score; APACHE, acute physiology and chronic health evaluation; Lac, lactate; CRP, C-reactive protein; PCT, procalcitonin; ALB, albumin; eGFR, estimated glomerular filtration rate; sCR, serum creatine; SD, standard deviation; IQR, interquartile range.
Consistency across the different standards in diagnosing AKI
The number of patients with AKI was the highest under the pRIFLE standard (46 cases). Five patients with sepsis who were not diagnosed with AKI according to both the KDIGO and pROCK standards were diagnosed with AKI according to the pRIFLE standard, four of whom were diagnosed with AKI stage 1 and one with AKI stage 2. The pROCK standard diagnosed the fewest number of AKI cases (34 cases). Only one patient was not diagnosed with AKI under the pRIFLE standard who was diagnosed by the KDIGO and pROCK standards, with both indicating AKI stage 3. The consistency of diagnosing SA-AKI across the pRIFLE, KDIGO, and pROCK standards was good (all P values <0.05; Table 4). The consistency of diagnosing staging between the pRIFLE and KDIGO was good (κ=0.0671; κ>0.60), while that between the RIFLE and pROCK was poor (κ=0.328).
Table 4
| Classification | pROCK | KDIGO |
|---|---|---|
| pRIFLE | 0.526 | 0.766 |
| pROCK | – | 0.733 |
| P | <0.001 | <0.001 |
AKI, acute kidney injury; pROCK, Pediatric Reference Change Value Optimized for AKI; KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease.
Risk factors for AKI in children diagnosed with sepsis
Children who met any of the diagnostic criteria of the three standards were analyzed for AKI risk factors. We established a multiple-factor logistic regression analysis based on a single-factor model, which showed that a high PRISM scores and a high PCT level were independent risk factors in across all three standards. In addition, in the pRIFLE criteria, combined elevated lactic acid was also an independent risk factor; in the KDIGO criteria, high MODS scores and low MAP were independent risk factors; in the pROCK criteria, low ALB was an independent risk factor (Table 5).
Table 5
| Independent variables | P | OR | 95% CI |
|---|---|---|---|
| pRIFLE | |||
| PCT | 0.02 | 1.106 | 1.015–1.207 |
| Lac | 0.03 | 0.221 | 0.055–0.885 |
| PRISM score | 0.02 | 0.223 | 0.064–0.774 |
| KDIGO | |||
| MAP | 0.03 | 0.804 | 0.657–0.983 |
| MODS score | 0.03 | 2.232 | 1.086–4.587 |
| PRISM score | 0.02 | 0.749 | 0.586–0.956 |
| PCT | 0.02 | 1.32 | 1.045–1.669 |
| pROCK | |||
| PRISM score | 0.008 | 2.14 | 1.222–3.747 |
| PCT | 0.003 | 1.329 | 1.235–1.745 |
| ALB | 0.02 | 0.924 | 0.834–1.224 |
| Mortality | |||
| CRRT | 0.03 | 14.404 | 1.394–148.831 |
| Septic shock | 0.002 | 3.5 | 2.483–53.659 |
SA-AKI, sepsis-associated acute kidney injury; OR, odds ratio; CI, confidence interval; pRIFLE, Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease; PCT, procalcitonin; Lac, lactate; PRISM, pediatric risk of mortality; KDIGO, Kidney Disease Improving Global Outcomes; MAP, mean arterial pressure; MODS, multiple organ dysfunction score; pROCK, Pediatric Reference Change Value Optimized for AKI; ALB, albumin; CRRT, continuous renal replacement therapy.
Prediction of SA-AKI
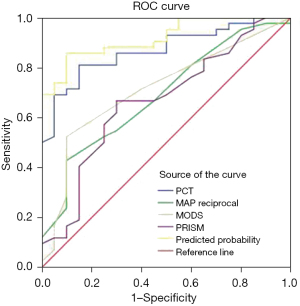
ROC curve analysis was performed on PCT level, PRISM score, MODS score, and MAP, and it was found that PCT level had predictive value in all three diagnostic standards. The predicted area under the ROC curve (AUC) of PCT level under the pRIFLE, KDIGO, and pROCK standards were 0.819, 0.870, and 0.728, respectively, with a sensitivity and specificity of 60.9% and 87.5%, 81% and 85%, 61% and 85%, respectively. An elevated PCT level had the largest AUC in the KDIGO criteria, with the optimal cutoff point being 7.485 ng/mL (Table 6). Analysis of the combined MAP score, PRISM score, and other indicators, suggests that the predictive value and sensitivity of multiple indicators used in combination are higher than those of any indicator used alone (Table 6, Figure 1).
Table 6
| Indicator | Cut-off | Sensitivity (%) | Specificity (%) | AUC | 95% CI | P |
|---|---|---|---|---|---|---|
| PCT | 7.485 ng/mL | 81 | 85 | 0.87 | 0.782–0.957 | <0.001 |
| MAP reciprocal | 0.0204 mmHg | 42.9 | 90 | 0.688 | 0.550–0.826 | 0.02 |
| MODS | 9.5 | 52.4 | 90 | 0.714 | 0.578–0.849 | 0.007 |
| PRISM | 19.5 | 66.7 | 70 | 0.666 | 0.854–0.984 | <0.001 |
| Union | – | 85.7 | 90 | 0.919 | 0.854–0.984 | <0.001 |
PCT, procalcitonin; MAP, mean arterial pressure; MODS, multiple organ dysfunction score; PRISM, pediatric risk of mortality; SA-AKI, sepsis-associated acute kidney injury; AUC, area under curve; CI, confidence interval.
SA-AKI mortality analysis
By the follow-up 28 days after admission, seven children had died. According to the pRIFLE standard, there were seven deaths in the AKI group (accounting for 15.2% of the total number of those with AKI) and no deaths in the non-AKI group. According to the KDIGO standard, there were seven deaths in the AKI group (accounting for 16.7% of the total number of those with AKI groups) and no deaths in the non-AKI group. According to the pROCK standard, there were six deaths in AKI group (accounting for 17.6% of the total number of those with AKI) and one death in the non-AKI group (accounting for 3.6% of the total number of non-AKI cases). There was a significant difference in the mortality rate between the AKI group and the non-AKI group across the standards.
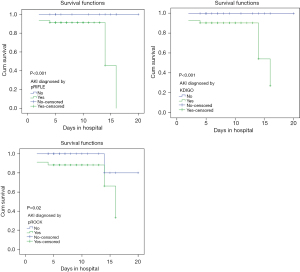
The children were divided into two groups: non-AKI and AKI stage 1 cases were placed in the mild/no-AKI group, while cases of AKI stages 2 and 3 were placed in the severe AKI group. According to the pRIFLE, KDIGO and pROCK standard, the mortality rate of the mild/no-AKI group was 7.14%, 5.56% and 5.13%, respectively; and that of severe AKI group was 20%, 19.23% and 21.73%. These results indicate that the mortality rate increased as the staging degree increased. Using death as an endpoint, a survival analysis graph was drawn (Figure 2), which demonstrated that the risk of the AKI group was significantly higher than that of the non-AKI group. Because there were deaths in both groups when pROCK was used, Cox regression was performed [hazard ratio (HR) 5.756, 95% CI: 0.689–48.053], but the sample size was too small, the confidence interval was too wide, and the statistical power was low.
We divided the children into a death group and survival groups in a cohort study and included all potential confounding variables [univariate logistic regression analysis P<0.05; age, shock, PCT level, renal replacement therapy (RRT), PRISM score] into a multivariate model to evaluate curve differences. It was found that shock and whether RRT was performed were independent risk factors for death and were closely associated with mortality (Table 5).
Discussion
Sepsis is an important cause of AKI in patients in the PICU, with an incidence of about 10% to 55% (5,10,11). The incidence rate SA-AKI in this study was slightly higher than this standard, which may be related to factors such as the severity of the critically ill children admitted to this study center and the small sample size. It may also indicate that the incidence of AKI in children across different studies may vary due to differences in study duration, case combination, and definitions adopted.
There is a lack of consensus concerning which scoring standards should be used to assess critically ill children. Although the introduction of different AKI diagnostic criteria is conducive to the more accurate prediction of prognosis of different AKI populations, it makes diagnosing AKI in clinic difficult and the evaluation of the results of studies on diagnosing AKI becomes challenging. Some researchers (12-14) believe that the KDIGO standard is more suitable. Meanwhile, Wei et al. (15) compared the diagnostic and predictive values of the KDIGO and pROCK standards for AKI in PICU and found that the predictive value of the pROCK standard for mortality rate of these children was slightly better than that of the KDIGO standard. Gao et al. (16) examined the incidence of AKI after cardiac surgery and found that the agreement between pRIFLE and KDIGO was almost perfect. Ozkaya et al. (17) analyzed the research on the incidence of AKI in children with sepsis in the PICU using the pRIFLE, KDIGO, and Acute Kidney Injury Network (AKIN) criteria, and concluded that the pRIFLE criteria are the most suitable. In our study, diagnosis of SA-AKI according to the pRIFLE, KDIGO, and pROCK standards yielded significantly different incidences. Although the overall consistency of diagnosing SA-AKI with the three standards was good and there was no statistical difference, there were differences in staging consistency. This suggests that there are discrepancies in staging results and incidences when different standards are used to diagnose the same patient. We should examine the possible impact of the use of these different AKI diagnostic criteria on research results for different populations.
The incidence and specificity of the three standards in this study also diverged. The incidence of SA-AKI in the pROCK group (35/62, 56.5%) was lower than that in the other two groups, indicating that pROCK may have higher specificity for diagnosing SA-AKI and may help reduce overdiagnosis, which is consistent with the findings of Zeng et al. (18). In this study, the critical scores of children in the pROCK group, especially the PRISM score, was significantly higher than that in other two groups. The rate of CRRT treatment (41.2%) and the proportion of mechanical ventilation use were significantly higher than those in the other two groups, indicating that this diagnostic standard may have a good correlation with patient illness severity, mechanical ventilation use, and CRRT treatment. It is necessary to closely monitor the major changes of kidney-related indicators during treatment in order to achieve early diagnosis and treatment. This conclusion is consistent with other related study (18). The incidence of SA-AKI in children and mortality rate (3/62) in the pRIFLE group were the highest. This indicates that pRIFLE is more sensitive in the early detection of AKI, which is in line with results previously published by other authors (17). Because AKI stage 1 had the greatest difference in diagnosis incidence, but the sCR level does not rise until renal reserve has been removed, overemphasis on diagnostic specificity may lead to underdiagnosis of children at risk for AKI (19). Therefore, it is critical to adopt the most sensitive and specific diagnostic criteria for the combined diagnosis of PICU children with sepsis. Considering that there was no statistical difference in SA-AKI incidence between the pRIFLE and KDIGO standards and there was good consistency in diagnosis staging, we believe that it is equally important to combine pROCK with pRIFLE or KDIGO.
The mechanism of severe SA-AKI is related to changes in blood flow dynamics, renal tubular injury, ischemia-reperfusion, and systemic inflammatory response. The lower the PCIS score, the higher the MODS score, PRISM score and APACHE score, the more severe organ function impairment in sepsis children, and the more likely it is to develop AKI. The MAP of patients with AKI was lower than that of those without it. A study has shown that when MAP <73 mmHg, there is a risk of AKI because it can cause dilation of the afferent arterioles and efferent arterioles of the kidney glomerulus, resulting in a decrease in the glomerular filtration rate. Even if renal blood flow perfusion is not reduced, renal function can further deteriorate (20). Shock and a high lactic acid level indicate reduced renal perfusion, which affects the reduction of glomerular blood flow and the glomerular filtration rate, which leads to AKI. Our study showed that shock at admission, low MAP, low PCIS score, high MODS score, high PRISM score, high APACHE score, high lactate level, low ALB level, and high PCT level significantly increased the risk of SA-AKI.
We also found that high PRISM and MODS scores, high PCT level, high arterial blood lactate level, low MAP, and hypoalbuminemia were risk factors for SA-AKI in children in the PICU. Among the three standards, high PRISM scores and elevated PCT level were independent risk factors for SA-AKI. KDIGO had more independent risk factors, which is consistent with similar research (21,22). The PRISM score is currently the most widely used assessment scale in the PICU and shows good ability to predict the severity and mortality risk of critically ill children (21). PCT levels can determine the severity of sepsis with high accuracy. High levels indicate more severe infection, which can cause bone marrow hematopoietic function to be suppressed, a large number of microthrombi to form, and the excessive consumption of platelets, which induce the reduction of glomerular blood flow and the decline of glomerular filtration rate, ultimately leading to AKI (23). Clinicians can identify potential SA-AKI in patients according to the above results and implement relevant treatments to reduce the incidence of AKI. The AUC results of this study show that prioritizing the evaluation of PCT level, PRISM score, MODS score, and MAP, among others, can shorten the diagnosis time and improve efficiency.
Regardless of the diagnostic criteria used, the mortality rate of SA-AKI in the PICU reaches as high as 64% (2). Our study demonstrated that regardless of the diagnostic criteria used, the mortality rate increases with the increase in SA-AKI staging. The risk of death from severe SA-AKI was more than twice that of non- or light AKI, which is consistent with other reports (5,15,22,24). Multivariate analysis showed that septic shock and the need for CRRT were independent risk factors for death. This may be explained as follows: first, during septic shock, tissue hypoperfusion is severe and cardiovascular dysfunction is obvious, which aggravates the underlying condition and increases the difficulty of treatment. Meanwhile, inflammation can promote platelet aggregation and activation, causing coagulation dysfunction, aggravating microthrombus formation and reducing prognosis. Second, patients in this study who required CRRT treatment had a higher mortality rate than other patients. This may be due to other organ failure, severe renal injury, and the inability to completely resolve the primary cause, leading to further aggravation of renal injury and the formation of a vicious cycle through interactions between the kidneys and other organs, ultimately affecting prognosis. CRRT represents an important advancement in the treatment of SA-AKI. Most studies have confirmed that it can improve the patient survival rate within 28 days (25-27). However, there is no uniform standard for treatment timing and course. For example, a study has observed that patients who start CRRT within 5 days of admission to the ICU for SA-AKI and within 24 hours of reduced UO have a better prognosis (25). Recently, Gaudry et al. (28) compared the 60-day mortality rate of early or delayed use of CRRT to treat SA-AKI and found no difference (P>0.05). However, due to the small sample size of this study and a lack of analysis for the timing or dose of CRRT, additional research is needed in the future.
This study has certain limitations which should be mentioned. First of all, patients who were hospitalized for less than 48 hours or died within 48 hours were excluded. These patients often have worse baseline conditions when admitted to hospital and are more likely to develop SA-AKI. This has probably decreased the number of children with SA-AKI included in the study, underestimating the true incidence. Due to the single-centre design, the preliminary results obtained will expand upon by increasing sample size in future multicentric studies. Second, children’s eGFR was estimated using the Schwartz formula; however, the validity of this formula for evaluating eGFR in Chinese children remains to be confirmed.
Conclusions
AKI incidence and mortality rates of children with sepsis are high and related to the severity of the child’s condition. The consistency of AKI diagnosis using the pRIFLE, KDIGO, and pROCK criteria in the same patient was good. pROCK may have higher specificity and better correlation with the severity of the child’s condition and poor prognosis, while pRIFLE may have better incidence of AKI. There are many independent risk factors for SA-AKI, and the clinical value of the combined prediction of multiple risk factors is high. Clinically, multiple diagnostic criteria should be used in combination, and additional attention should be paid to the monitoring and treatment of AKI in PICU children with sepsis. The early detection and timely and appropriate treatment can prevent and reverse the progression of renal failure.
Acknowledgments
We thank Dr. Carlos Delgado-Miguel (La Paz Children’s University Hospital, Madrid, Spain) for the critical comments and valuable advice on this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-34/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-34/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University (No. Quick-PJ-2023-16-10). All patient’s guardians consented to submission of this research to the journal and provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015;191:1147-57. [Crossref] [PubMed]
- Fitzgerald JC, Basu RK, Akcan-Arikan A, et al. Acute Kidney Injury in Pediatric Severe Sepsis: An Independent Risk Factor for Death and New Disability. Crit Care Med 2016;44:2241-50. [Crossref] [PubMed]
- Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007;71:1028-35. [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [Crossref] [PubMed]
- Xu X, Nie S, Zhang A, et al. A New Criterion for Pediatric AKI Based on the Reference Change Value of Serum Creatinine. J Am Soc Nephrol 2018;29:2432-42. [Crossref] [PubMed]
- Schlapbach LJ, Watson RS, Sorce LR, et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024;331:665-74. [Crossref] [PubMed]
- Kim IY, Kim JH, Lee DW, et al. Fluid overload and survival in critically ill patients with acute kidney injury receiving continuous renal replacement therapy. PLoS One 2017;12:e0172137. [Crossref] [PubMed]
- Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med 2017;376:11-20. [Crossref] [PubMed]
- Xu X, Nie S, Zhang A, et al. Acute Kidney Injury among Hospitalized Children in China. Clin J Am Soc Nephrol 2018;13:1791-800. [Crossref] [PubMed]
- Bresolin N, Silva C, Halllal A, et al. Prognosis for children with acute kidney injury in the intensive care unit. Pediatr Nephrol 2009;24:537-44. [Crossref] [PubMed]
- Shen H, Qu D. Risk factors of septic shock with acute kidney in 89 children. Chin Pediatr Emerg Med 2019;26:666-70.
- Kuai Y, Li M, Chen J, et al. Comparison of diagnostic criteria for acute kidney injury in critically ill children: a multicenter cohort study. Crit Care 2022;26:207. [Crossref] [PubMed]
- Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 2015;10:554-61. [Crossref] [PubMed]
- Luo X, Jiang L, Du B, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care 2014;18:R144. [Crossref] [PubMed]
- Wei C, Hongxia G, Hui F, et al. Impact of and risk factors for pediatric acute kidney injury defined by the pROCK criteria in a Chinese PICU population. Pediatr Res 2021;89:1485-91. [Crossref] [PubMed]
- Gao P, He W, Jin Y, et al. Acute kidney injury after infant cardiac surgery: a comparison of pRIFLE, KDIGO, and pROCK definitions. BMC Nephrol 2023;24:251. [Crossref] [PubMed]
- Ozkaya PY, Taner S, Ersayoğlu I, et al. Sepsis associated acute kidney injury in pediatric intensive care unit. Ther Apher Dial 2023;27:73-82. [Crossref] [PubMed]
- Zeng J, Miao H, Jiang Z, et al. Pediatric Reference Change Value Optimized for Acute Kidney Injury: Multicenter Retrospective Study in China. Pediatr Crit Care Med 2022;23:e574-82. [Crossref] [PubMed]
- Goldstein SL. A New Pediatric AKI Definition: Implications of Trying to Build the Perfect Mousetrap. J Am Soc Nephrol 2018;29:2259-61. [Crossref] [PubMed]
- Katayama S, Nunomiya S, Koyama K, et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care 2017;21:229. [Crossref] [PubMed]
- Gonçalves JP, Severo M, Rocha C, et al. Performance of PRISM III and PELOD-2 scores in a pediatric intensive care unit. Eur J Pediatr 2015;174:1305-10. [Crossref] [PubMed]
- White KC, Serpa-Neto A, Hurford R, et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med 2023;49:1079-89. [Crossref] [PubMed]
- Cui N, Zhang H, Chen Z, et al. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Med Res 2019;47:1573-9. [Crossref] [PubMed]
- Cobussen M, Verhave JC, Buijs J, et al. The incidence and outcome of AKI in patients with sepsis in the emergency department applying different definitions of AKI and sepsis. Int Urol Nephrol 2023;55:183-90. [Crossref] [PubMed]
- Baek SD, Yu H, Shin S, et al. Early continuous renal replacement therapy in septic acute kidney injury could be defined by its initiation within 24 hours of vasopressor infusion. J Crit Care 2017;39:108-14. [Crossref] [PubMed]
- Bottari G, Guzzo I, Marano M, et al. Hemoperfusion with Cytosorb in pediatric patients with septic shock: A retrospective observational study. Int J Artif Organs 2020;43:587-93. [Crossref] [PubMed]
- Brouwer WP, Duran S, Kuijper M, et al. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care 2019;23:317. [Crossref] [PubMed]
- Gaudry S, Hajage D, Schortgen F, et al. Timing of Renal Support and Outcome of Septic Shock and Acute Respiratory Distress Syndrome. A Post Hoc Analysis of the AKIKI Randomized Clinical Trial. Am J Respir Crit Care Med 2018;198:58-66. [Crossref] [PubMed]


