
Eosinophilic esophagitis: absolute eosinophilic count, peak eosinophilic count, and potential biomarkers of eosinophilic degranulation products—an in-depth systematic review
Highlight box
Key findings
• Eosinophilic degranulation ratio in the esophagus may be critical for evaluating properly the biomarkers of eosinophilic esophagitis.
What is known and what is new?
• Eosinophilic esophagitis is a challenging relapsing disease.
• A few minimally invasive methods and biomarkers may be suggested as alternative tools in diagnosing and monitoring this esophagitis.
What is the implication, and what should change now?
• Potential biomarkers should be monitored using the proposed eosinophilic degranulation ratio.
Introduction
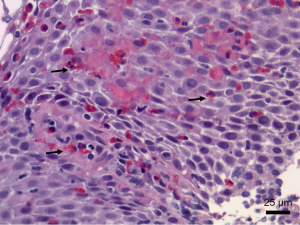
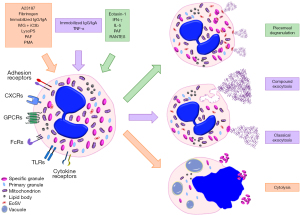
Eosinophilic esophagitis is a chronic inflammatory disorder, often relapsing. It is closely associated with excessive mucosal eosinophilic infiltration due to disturbance in immunological response involving eosinophils and other inflammatory blood cells and their secretory granules. A clinicopathological diagnosis recommends that a minimum of 15 eosinophils per high-power field, among other supporting histological features, are required for the diagnosis (1,2). Current recommendations require endoscopic biopsies from at least two sites and a minimum of five biopsy fragments because of the uneven distribution of eosinophils along the esophagus (1). Other histological features that support the diagnosis include surface layering of eosinophils, eosinophilic micro-abscesses, marked basal cell hyperplasia, and potential subepithelial fibrosis of the lamina propria, which is often a rare complication of eosinophilic esophagitis with poor response to treatment (Figure 1). It has been shown from a study that disease severity, as well as outcome, is directly proportional to the degree of mucosal infiltration by eosinophils, and this further affirms their critical roles in eosinophilic esophagitis (3). Etiological factors have been linked with an abnormal immune response to some diets or other environmental factors. For example, a study reported that 86% of their study population had food allergies while 65% had a background history of allergic conditions like asthma (4). Reports of a complex interplay of environmental and genetic factors may result in eosinophilic degranulation in the esophagus and subsequent immune-inflammatory response that culminates in esophageal mucosal damage (5). Consequently, a good understanding of the biology of eosinophil degranulation, its biological functions, and potential inhibitors is essential for new diagnostic methods, treatment options, and disease monitoring (6), particularly considering how rural health disparities may influence prevalence data in pediatric eosinophilic esophagitis (7). In Figure 2, the pathogenesis of degranulation is depicted.
Clinical symptoms and signs at diagnosis vary with age from different studies. Epigastric pain was found in a study as the most common symptom and, dysphagia as the least common, while an intermediate number of patients presented with heartburn (4). In another study, food impaction and choking were the most common symptoms among a study population reporting a significant rise in eosinophilic esophagitis in Korea from 2006 to 2017 (8). There was also a reported increase in the incidence of eosinophilic esophagitis in another study population (9). Indeed, some previous studies in searching histopathologic markers of progression have been fruitful, but clinical trials have not started properly yet (10-12). Essentially, the number of studies on symptoms and their variation is very large and involves several investigations across age groups and the readers may consider evaluate some recent references (9,11,13-19). Now, diagnosis and monitoring require invasive procedures like endoscopy. This poses a potential risk of complications and cost implications for these patients and the national government or healthcare providers. This is made worse due to the need for repeating endoscopic procedures for disease monitoring to assess the degree of response to treatment. Consequently, there is an increasing need to develop new alternative diagnostic and monitoring methods urgently. Samples may be obtainable through minimally-invasive procedures that include body fluids like blood, urine, and mucosal secretory fluids via processes that include esophageal string tests, cytosponge, trans-nasal endoscopy, and endoFLIP (20,21). It is important to emphasize that some procedures may be considered invasive in specific age groups. For this purpose, various biomarkers, including eosinophil and non-eosinophil degradation products like blood eosinophil-derived neurotoxin (EDN) and eotaxin-3, have been shown to potentially correlate with peak eosinophil counts (9). In another study, eosinophil peroxidase (EPO) sampled via esophageal brushing with subsequent assay showed sensitivity and specificity of close to 100% each when compared with peak eosinophil counts in tissue biopsies (22). These recent findings of potentially useful diagnostic and monitoring biomarkers, if replicated in other studies and approved, will hopefully help to bridge the knowledge gap in achieving non-invasive or minimally invasive methods and overcome some initial attempts or deadlocks (23-25).
This study uses a systematic approach to identify and document the types and roles of potential biomarkers in eosinophilic esophagitis, including eosinophil and non-eosinophil degranulation products, and the ratio of degranulated eosinophils over all eosinophils, including degranulated and granulated cells. Identifying and defining specific biomarkers could be a good premise to launch a search for potential inhibitors that may become useful in treating and managing eosinophilic esophagitis. These biomarkers will also be valuable tools for measuring disease activity (26). We present this article in accordance with the PRISMA reporting checklist (27) (available at https://tp.amegroups.com/article/view/10.21037/tp-23-478/rc).
Methods
This study aims to identify and discuss the potential roles of some newly described eosinophilic and non-eosinophilic degranulation products, which are primarily obtainable via non to minimally invasive methods, using a systematic review of recent studies.
Eligibility criteria and literature search
Systematic reviews are gold standards in public health, but they may not be qualified as PRISMA-based systematic reviews sometimes due to the heterogeneity of the retrieved studies. Despite the approach and the organic structure used are solid, some limitations are unavoidable and will be highlighted further below. Articles were included based on diagnostic criteria used in the study. Other inclusion criteria include new biomarkers, which can be sampled through non or minimally invasive procedures. Study designs range from cross-sectional studies to retrospective studies, and cohort studies. Studies that do not include eosinophil degranulation products or eosinophilic esophagitis were excluded. We systematically reviewed English-language articles using PubMed, Scopus, Medline, Google Scholar, and Cochrane Database with the assistance of an experienced statistician from January 2011 to December 2022. Search criteria include eosinophil*, esophagus*, degranulated, granulated, peripheral blood marker, biomarker, brush, string test, minimally invasive, semi-invasive, brush, and assay. Articles that fulfill the above inclusion criteria and contain all search elements were selected and duplicates were removed. All abstracts were retrieved and downloaded, and a further search was made to retrieve full articles that did not contain a PDF version in the initial search.
Study selection
The first author and the senior author reviewed all abstracts independently and included only articles that met all inclusion criteria. All abstracts that contain non-desired items like reflux esophagitis or asthma were excluded from the study. The same authors retrieved and reviewed the full PDF copies of selected articles. All PDF copies of the articles were further examined and perused in detail. A consensus among all three authors was reached.
Data collection
We independently reviewed full PDF articles and extracted data on a Microsoft Excel spreadsheet. These data were subsequently examined, and a consensus was reached. Any discrepancy was resolved through agreement. The data spreadsheet includes names of lead authors, year of publication, age of study participants, sample size, use of control population or not, sample collection method, list of biomarkers, study design, biomarker detection method, and study outcome. Similar parameters were mostly included in other studies (28-30). Table 1 provides the details of the studies investigated in this review.
Table 1
| Main author [year] (Ref) | Age (years), range or mean ± SD | N | Geo | Study | Ctrl. | Method | Biomarkers tested | Detection method | Statistically significant biomarkers | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Avinashi [2020] (23) | 4–17 | 43 | Canada | CSPS | Yes | OPS, EB | EDN, EPO, MBP-1, IL-5, IL-8, IL-13 | ELISA | EDN and EPO with PEC | Oropharyngeal swab assay not useful for diagnosis or monitoring |
| Carrasco [2017] (4) | 1–14 | 14 | Brazil | CSPS | No | EB | Eos granules | LM | Eos granules | Eos granules are present in up to 100% of EoE |
| Cengiz [2019] (30) | 18–46 | 29 | Turkey | CSPS | Yes | AEC, PEC | ECP | Immunoassay | ECP | ECP has high sensitivity and specificity for EoE and correlates with symptoms |
| Kim [2019] (8) | 46.2±14.4 | 72 | South Korean | RS | No | EB | EDN, eotaxin-3, tryptase | Immunoassay | EDN-eotaxin-3 with PEC, EDN-tryptase with EoE score | Tryptase, EDN, and eotaxin-3 levels in esophageal biopsy specimens could be promising biomarkers |
| Lu [2018] (31) | 11.2±1.3 | 31 | USA | CSPS | Yes | PB | HETE, AEC, cytokines | Immunoassay | HETE | Significant correlation between AEC and HETE |
| Peterson [2019] (29) | 19–74 | 34 | USA | Cohort | No | EB | MBP-1 | IF | MBP-1 | MBP-1 correlates with symptoms and may measure disease activity |
| Saffari [2016] (22) | n.a. | 36 | USA | CSPS | Yes | EB and OPS | EPO | SPA | EPO in brushings | EPO correlates with PEC; it can detect and monitor EoE activity |
| Schoepfer [2018] (32) | 43.5±15.7 | 200 | Switzerland | CSPS | Yes | EB | Eos granules | LM | Eos granules | Eos degranulation correlates with sub-epithelial Eos count and disease activity |
| Sridhara [2012] (33) | 22–47 | 30 | USA | RS | Yes | EB | EDN, MBP-1, tryptase | IF | Tryptase | Tryptase was higher in EoE than GERD and BE, unlike EDN/MBP-1 |
| Wechsler [2021] (34) | 8.8 | 41 | USA | Cohort | Yes | Blood, urine | EDN, MBP-1, AEC | ELISA | AEC, CLC/GAL-10, ECP, EDN, OPN, MBP-1 | AEC, CLC/GAL-10, ECP, EDN, OPN, and MBP-1 are superior to AEC alone in the diagnosis of EoE |
SD, standard deviation; n.a., not available; Geo, geographical area; CSPS, cross-sectional prospective study; RS, retrospective study; Ctrl., controls; OPS, oropharyngeal swab; EB, endoscopic (esophageal) biopsy; AEC, absolute eosinophilic count; PEC, peak eosinophilic count; PB, peripheral blood; EDN, eosinophil derived neurotoxin; EPO, eosinophil peroxidase; MBP-1, major basic protein 1; IL-5, interleukin 5; IL-8, interleukin 8; IL-13, interleukin 13; ECP, eosinophilic cationic protein; HETE, 15(S)-hydroxyeicosatetraenoic acid; ELISA, enzyme-linked immunosorbent assay; LM, light microscopy; IF, immunofluorescence; SPA, spectrophotometric absorbance; EoE, eosinophilic esophagitis; Eos, eosinophilic; CLC/GAL-10, Charcot-Leyden crystal protein/galectin-10; OPN, osteopontin; GERD, gastro-esophageal reflux disease; BE, Barrett esophagitis.
Results
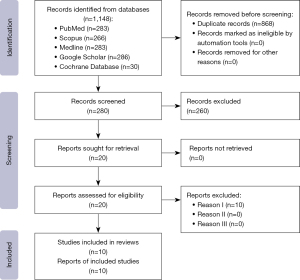
An initial search on Scopus and other online databases yielded 286 articles, out of which 20 were selected after abstract review, and these were further reviewed in full text. Consequently, ten articles were selected and included in this review.
All duplicates were removed from abstracts and full articles. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowsheet for data is presented in Figure 3.
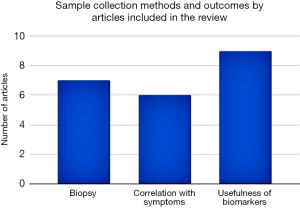
Most of the articles were published in or after 2016, within the second half of our pre-approved study period. Four of the studies were done in a pediatric population, five in the adult population, and one did not mention the age group of the study population in detail. Sample collection methods range from esophageal biopsies, combined esophageal biopsy and oropharyngeal swab, combined esophageal biopsy, and brushing, peripheral blood, a combination of esophageal biopsy, blood, and swab, and a combination of blood and urine (Table 1).
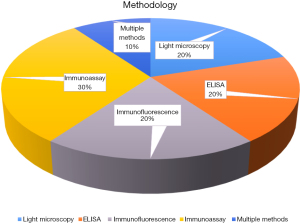
Most of the studies involved using controls (9 out of 10), while only one did not include the use of the control population. EDN and major basic protein 1 (MBP-1) were the most common biomarkers tested (4). EPO was tested in two studies, while other studies involved various combinations of multiple biomarkers, including absolute eosinophil count (AEC), eotaxin-3, eosinophilic cationic protein (ECP), and un-specified granule proteins. Immunoassay/immunofluorescence was the preferred method for biomarker measurement (4), two studies each used the ELISA method, and one was through spectrophotometry. Figure 4 shows the frequency of detection methods by articles reviewed.
Study outcome varies based on the type of biomarker tested for and if there was a test of association or significance and the interest in the literature is depicted (Figures 5,6). In one study, oropharyngeal swab biomarker testing for EPO, MBP-1, and EDN showed no significant correlation with peak eosinophil count, unlike when these biomarkers were assayed from samples that were obtained from esophageal mucosal biopsies. Some of the other studies showed their ability to detect eosinophil and non-eosinophil degranulation products, including blood/urine MBP-1, EDN, ECP, 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE], EPO, and absolute eosinophil count, thereby serving as potential surrogates in making a diagnosis or monitoring disease progression. In particular, 15(S)-HETE may participate in the dysregulated immune response which characterizes eosinophilic esophagitis. However, 15(S)-HETE alone is no better than AEC and Th2 cytokines as a noninvasive means to distinguish eosinophilic esophagitis from other gastrointestinal conditions. There may be some role for this novel marker in combination with other peripheral markers, such as EDN, eotaxin-3, and IL-13, in the diagnosis and management of eosinophilic esophagitis (31). The prediction of histologic changes with biomarkers and subepithelial remodelling may be quite challenging (32-34).
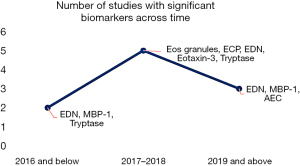
In another study, a random combination of any of these biomarkers with peripheral blood AEC was superior to only AEC (8). This combined method distinguishes successfully eosinophilic esophagitis from controls and correlates with histologic peak esophageal eosinophil counts (8). Similarly, MBP-1 shows a predictive role in diagnosis, unlike eotaxin-3, which was predictive of disease progression, and these findings further strengthen the need for a combination of biomarkers to be able to achieve positive multiplicative advantage (8,9). In a study, although the use of oropharyngeal swabs to collect samples for biomarker assay was easy and convenient, there was no evidence of a correlation between oral or oropharyngeal biomarkers and peak eosinophil count in the esophageal biopsy (23).
Quality assessment
Among different paper evaluation systems (JADAD, Delphi, CONSORT, and Cochrane Collaboration), we opted for the systematic review method with an “ad hoc” assessment. Differences in baseline features between groups, identification of allocation concealment, and dropout rates were used to evaluate the study quality. All included studies were considered harboring a satisfactory quality. All included studies were properly part of this systematic review.
Discussion
Eosinophilic esophagitis remains a puzzling disease that strongly needs further clarification on biomarkers and clinical standpoints (2,10-12,35-37). Although recently characterized as a distinct disease entity, eosinophilic esophagitis has held increasing incidence, especially in countries where it was previously described as a rarity. There is a disproportionately higher incidence in urban compared to rural areas. A study has suggested unequal distribution and easy accessibility of healthcare services as possible reasons (7). Kim et al. reported a significant rise in eosinophilic esophagitis in Korea from 2006 to 2017 (8). There was also a reported increase in the incidence of eosinophilic esophagitis in another study population (9). Our previous studies in searching histopathologic markers of progression have been fruitful, but clinical trials have not properly started yet (10-12). The utility to explore non-invasive diagnostic markers and monitoring tools for eosinophilic esophagitis has also been recently emphasized in a study on the age variation of eosinophilic esophagitis. The clinical presentation of this disease varies among different age groups, but the diagnostic criteria and therapeutic goals remain similar for both pediatric and adult groups (38). Ten studies were selected from an initial search of 286 based on the chosen criteria. The tested biomarkers include eosinophilic and non-eosinophilic granule proteins, AEC, and peak eosinophil count. Most studies that tested for blood, serum, and urine biomarkers found a significant association with clinical status or peak eosinophil count. However, another study on oropharyngeal swab biomarkers showed no association with clinical level or peak eosinophil count. Following diagnosis, treatment methods vary, from dietary restriction to antibodies against eosinophil degranulation and inflammatory products. A study demonstrates significant disease control, clinically and histologically, by treating with steroid fluticasone propionate (24). Another study reported that an antibody to IL-5, mepolizumab, reduced intraepithelial eosinophils and improved endoscopic and histological findings among children with eosinophilic esophagitis (25).
Our study has identified preliminary findings through a systematic review of the most recent articles on using minimally invasive methods and biomarkers that may be useful as alternative tools in diagnosing and monitoring eosinophilic esophagitis. A few minimally invasive techniques and biomarkers may be helpful as alternative tools in diagnosing and monitoring eosinophilic esophagitis. While there is no consensus on the clinical usefulness of these biomarkers and sampling methods, our review has identified a paucity of in situ eosinophilic scores. We suggest that the degranulation ratio (DGE/DGE + NDGE) may be critical for evaluating these biomarkers. An increasing trend may culminate in the potential clinical use of clinical biomarkers upon approval by regulatory bodies in making future diagnoses. Our study affirms a recent surge in studies that seek to discover and characterize specific biomarkers and minimally invasive sample collection methods, as more than half of our reviewed articles were published in the most recent 6 years. Also, more of these studies were conducted in adults than children. The three most useful biomarkers have been shown to be EDN, MBP-1, and EPO, and these are more sensitive when combined with peripheral blood absolute eosinophil count. While there is no consensus on the clinical usefulness of these biomarkers and sampling methods, this study has identified an increasing trend that may culminate in their potential clinical use, upon approval by regulatory bodies, in making or monitoring eosinophilic esophagitis in the future.
This review may not reach the level of a PRISMA-based systematic review, because the studies taken into consideration are heterogenous, as displayed in our table with geographical areas and methodology used. Nevertheless, it is a solid review that may highlight the importance of biomarkers and, probably, the necessity to score the esophageal biopsies with both a peak eosinophilic count and a score of the degranulated eosinophils (degranulated eosinophils/degranulated eosinophils and granulated eosinophils). It is well known that there is a (I) risk of bias common to several studies, such as lack of blinding for subjective outcomes or unavailability of comprehensive data; (II) inconsistency of association or effect, as shown by high heterogeneity; (III) imprecision due to small sample size (the inclusion of such studies may be questionable, but there are numerous publications advocating to not eliminate studies only because the events under examination are few); (IV) indirectness of the clear-cut evidence, such as use of an intermediate or short-term outcome; and (V) likelihood of publication bias, as stated in Clarivate or Scopus guidelines. These limitations are paramount factors used to evaluate the level of evidence, but they may also be imperfect.
Conclusions
Overall, several identified clinically useful biomarkers and minimally invasive methods exist. Eosinophils and their products are more concentrated in tissues from esophageal biopsies than in body fluids and non-esophageal mucosal tissue, where they are often present in lower but assay-detectable concentrations. There is a need for more studies to improve our understanding of the proper monitoring of this disease. We confirm the clinical utilities of these biomarkers and minimally invasive methods in our quest to ease patients’ discomfort and save costs. Currently, there is a necessity to advance research optimizing diagnostic strategies, tailoring therapeutical approaches, safely monitoring of patients no matter the age is, and improve long-term outcomes trying to avoid the rare postulated fibrosis of the submucosa with unavoidable chronicity of the disease. We strongly advocate for the necessity to score the esophageal biopsies with both a peak eosinophilic count and a score of the degranulated eosinophils (degranulated eosinophils/degranulated eosinophils and granulated eosinophils) and having an experienced gastrointestinal pathologist is probably crucial for further evaluation of potential biomarkers in blood.
Acknowledgments
We acknowledge the Pathological Society of Great Britain and Ireland in supporting this academic activity.
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-478/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-478/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-478/coif). C.M.S. serves as an unpaid editorial board member of Translational Pediatrics from March 2022 to February 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonsalves N, Policarpio-Nicolas M, Zhang Q, et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006;64:313-9.
- Sergi C. Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr 2015;61:529-30.
- O'Shea KM, Rochman M, Shoda T, et al. Eosinophilic esophagitis with extremely high esophageal eosinophil counts. J Allergy Clin Immunol 2021;147:409-412.e5. [Crossref] [PubMed]
- Carrasco AEAB, Machado RS, Patrício FRDS, et al. Histological features of eosinophilic esophagitis in children and adolescents. Arq Gastroenterol 2017;54:281-5. [Crossref] [PubMed]
- Kottyan LC, Parameswaran S, Weirauch MT, et al. The genetic etiology of eosinophilic esophagitis. J Allergy Clin Immunol 2020;145:9-15. [Crossref] [PubMed]
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013;13:9-22. [Crossref] [PubMed]
- Sabet C, Klion AD, Bailey D, et al. Do rural health disparities affect prevalence data in pediatric eosinophilic esophagitis? J Allergy Clin Immunol Pract 2021;9:2549-51. [Crossref] [PubMed]
- Kim GH, Park YS, Jung KW, et al. An Increasing Trend of Eosinophilic Esophagitis in Korea and the Clinical Implication of the Biomarkers to Determine Disease Activity and Treatment Response in Eosinophilic Esophagitis. J Neurogastroenterol Motil 2019;25:525-33. [Crossref] [PubMed]
- Sarbinowska J, Wiatrak B, Waśko-Czopnik D. Searching for Noninvasive Predictors of the Diagnosis and Monitoring of Eosinophilic Esophagitis-The Importance of Biomarkers of the Inflammatory Reaction Involving Eosinophils. Biomolecules 2021;11:890. [Crossref] [PubMed]
- Garcia E, Ladak Z, Landry T, et al. Epithelial-mesenchymal transition, regulated by β-catenin and Twist, leads to esophageal wall remodeling in pediatric eosinophilic esophagitis. PLoS One 2022;17:e0264622. [Crossref] [PubMed]
- Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, et al. High Prevalence of Response to Proton-pump Inhibitor Treatment in Children With Esophageal Eosinophilia. J Pediatr Gastroenterol Nutr 2016;62:704-10. [Crossref] [PubMed]
- Persad R, Huynh HQ, Hao L, et al. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-α-NFκB pathway. J Pediatr Gastroenterol Nutr 2012;55:251-60. [Crossref] [PubMed]
- Bashaw H, Schwartz S, Kagalwalla AF, et al. Tutorial: Nutrition Therapy in Eosinophilic Esophagitis-Outcomes and Deficiencies. JPEN J Parenter Enteral Nutr 2020;44:600-9. [Crossref] [PubMed]
- Zammit SC, Cachia M, Sapiano K, et al. Eosinophilic gastrointestinal disorder: is it what it seems to be? Ann Gastroenterol 2018;31:475-9. [Crossref] [PubMed]
- Singla MB, Moawad FJ. An Overview of the Diagnosis and Management of Eosinophilic Esophagitis. Clin Transl Gastroenterol 2016;7:e155. [Crossref] [PubMed]
- Shah NA, Albert DM, Hall NM, et al. Managing eosinophilic esophagitis: challenges and solutions. Clin Exp Gastroenterol 2016;9:281-90. [Crossref] [PubMed]
- Schoepfer A, Safroneeva E, Straumann A. Eosinophilic Esophagitis: Impact of Latest Insights Into Pathophysiology on Therapeutic Strategies. Dig Dis 2016;34:462-8. [Crossref] [PubMed]
- Putnam PE. Esophagitis in Adolescents. Adolesc Med State Art Rev 2016;27:1-18.
- Murali AR, Gupta A, Attar BM, et al. Topical steroids in eosinophilic esophagitis: Systematic review and meta-analysis of placebo-controlled randomized clinical trials. J Gastroenterol Hepatol 2016;31:1111-9. [Crossref] [PubMed]
- Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults With Eosinophilic Esophagitis. Gastroenterology 2016;150:581-590.e4. [Crossref] [PubMed]
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3-20.e6; quiz 21-2. [Crossref] [PubMed]
- Saffari H, Leiferman KM, Clayton F, et al. Measurement of Inflammation in Eosinophilic Esophagitis Using an Eosinophil Peroxidase Assay. Am J Gastroenterol 2016;111:933-9. [Crossref] [PubMed]
- Avinashi V, Chan JM, Bush JW, et al. Poor Correlation of Oral Swabs with Esophageal Eosinophil Counts. Dysphagia 2020;35:773-9. [Crossref] [PubMed]
- van Rhijn BD, Verheij J, van den Bergh Weerman MA, et al. Histological Response to Fluticasone Propionate in Patients With Eosinophilic Esophagitis Is Associated With Improved Functional Esophageal Mucosal Integrity. Am J Gastroenterol 2015;110:1289-97. [Crossref] [PubMed]
- Assa'ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011;141:1593-604. [Crossref] [PubMed]
- Menard-Katcher C, Furuta GT. Non- and semi-invasive methods of monitoring eosinophilic esophagitis. Dig Dis 2014;32:102-6. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) 2021;74:790-9. [Crossref] [PubMed]
- Hines BT, Rank MA, Wright BL, et al. Minimally invasive biomarker studies in eosinophilic esophagitis: A systematic review. Ann Allergy Asthma Immunol 2018;121:218-28. [Crossref] [PubMed]
- Peterson KA, Gleich GJ, Limaye NS, et al. Eosinophil granule major basic protein 1 deposition in eosinophilic esophagitis correlates with symptoms independent of eosinophil counts. Dis Esophagus 2019;32:doz055. [Crossref] [PubMed]
- Cengiz C. Serum eosinophilic cationic protein is correlated with food impaction and endoscopic severity in eosinophilic esophagitis. Turk J Gastroenterol 2019;30:345-9. [Crossref] [PubMed]
- Lu S, Herzlinger M, Cao W, et al. Utility of 15(S)-HETE as a Serological Marker for Eosinophilic Esophagitis. Sci Rep 2018;8:14498. [Crossref] [PubMed]
- Schoepfer AM, Simko A, Bussmann C, et al. Eosinophilic Esophagitis: Relationship of Subepithelial Eosinophilic Inflammation With Epithelial Histology, Endoscopy, Blood Eosinophils, and Symptoms. Am J Gastroenterol 2018;113:348-57. [Crossref] [PubMed]
- Sridhara S, Ravi K, Smyrk TC, et al. Increased numbers of eosinophils, rather than only etiology, predict histologic changes in patients with esophageal eosinophilia. Clin Gastroenterol Hepatol 2012;10:735-41. [Crossref] [PubMed]
- Wechsler JB, Ackerman SJ, Chehade M, et al. Noninvasive biomarkers identify eosinophilic esophagitis: A prospective longitudinal study in children. Allergy 2021;76:3755-65. [Crossref] [PubMed]
- Rothenberg ME, Wen T, Greenberg A, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2015;135:500-7. [Crossref] [PubMed]
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2015;13:77-83.e2. [Crossref] [PubMed]
- di Pietro M, Fitzgerald RC. Research advances in esophageal diseases: bench to bedside. F1000Prime Rep 2013;5:44. [Crossref] [PubMed]
- Massironi S, Elvevi A, Panceri R, et al. Eosinophilic esophagitis: does age matter? Expert Rev Clin Immunol 2024;20:211-23. [Crossref] [PubMed]


