
Pediatric Ménière’s disease with disassociated cochlear and vestibular symptoms: a case report
Highlight box
Key findings
• In pediatric Ménière’s disease (MD), vestibular and cochlear symptoms may occur and progress independently. Hearing loss could lag behind the onset of vertigo episodes by several years in children with MD.
What is known and what is new?
• Caloric test seems more sensitive than video head impulse test for detecting MD. It is more likely to find abnormal results in cervical vestibular evoked myogenic potential than in ocular vestibular evoked myogenic potential in both pediatric and adult MD.
• We provide the thorough results of audiovestibular testings in a time line to follow how MD progressed in this child. This child could have attack of hearing loss even without vertigo in the progress of MD. And the reasons of dissociated vestibular and cochlear MD symptoms might be different between children and adults due to age-associated changes of balance function. Gadolinium-enhanced magnetic resonance imaging might also be sensitive in detecting endolymphatic hydrops of this child.
What is the implication, and what should change now?
• Apart from systemic administration of corticosteroids, postauricular subcutaneous administration might also be effective in pediatric MD during acute attacks. It is meaningful to pay attention to not only attack of vertigo but also the hearing loss with prolonging the follow-up period of pediatric patients with MD.
Introduction
It is widely believed that typical Ménière’s disease (MD) rarely occurs in childhood, with pediatric MD constituting a very small percentage (ranging from 1.5% to 3%) of all patients with MD (1-3). Moreover, MD comprises only 5% of children with dizziness diseases (4). The diagnosis of MD is relatively straightforward when symptoms are typical. Cases can be defined as either definite MD or probable MD according to the 2015 criteria specified jointly by the Classification Committee of the Bárány Society et al. (5). Compared to adults, children have inferior balance scores in tests such as sensory organization test (SOT) and computerized dynamic posturography (CDP) due to their insufficient vestibular system and central nervous system integration (6). It is reported that vestibular function might continue to mature at least until the ages of 15 to 17 years (7). For those certain pediatric patients who cannot describe their symptoms clearly or have atypical clinical symptoms, a thorough diagnosis of MD can be achieved through auxiliary tests, such as audiometry, vestibular tests, and imaging. However, previous pediatric MD case reports might have lacked some of the modern audiovestibular evaluation methods. In this report, we conducted thorough audiovestibular testing of the patient in temporal order to follow how vertigo and hearing loss progress in children with MD. We describe the case of a boy with MD who had disassociated symptoms of the vestibule and cochlear and had been admitted multiple times in Shandong Provincial ENT Hospital and had undergone comprehensive auxiliary tests, thus providing experience for the diagnosis and treatment of childhood MD. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-23/rc).
Case presentation
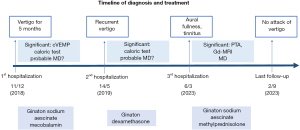
A 14-year-old boy was seen on 11 December, 2018 in Shandong Provincial ENT Hospital due to recurrent vertigo with left-sided tinnitus. He had firstly developed intermittent high-pitched tinnitus in the left ear for 7 months without provocation and recurrent vertigo associated with nausea and vomiting for 5 months. Accompanied with left-sided aural fullness, the frequency of attack of vertigo was once a month. The boy had no history of symptoms related to migraine or motion sickness. His parents denied any family history of vertigo disease. Although he had been administered with drugs to improve cerebral circulation at his local hospital previously, his experience of vertigo had persisted. The tympanic membranes were normal. The boy had no history of autoimmune-related symptoms and did not exhibit physical signs related to autoimmune diseases (such as joint pain, rubella, fever, etc.). Physical examination showed negative signs of spontaneous nystagmus, smooth pursuit, gaze nystagmus, Romberg, Mann, Fukuda, Dix-hallpike and Roll-test. Tympanometry showed A type in both sides. Auditory brainstem response (ABR) reflected differentiated I, III, V wave within normal latencies in both ears. Pure tone audiometry (PTA) reflected a normal hearing threshold in low and medium frequencies (Figure 1). Electrocochleography summating the potential/active potential (SP/AP) ratio was 0.14 of the left ear and 0.13 of the right. Caloric tests showed weakness of his left horizontal semicircular canal. Cervical vestibular evoked myogenic potentials (cVEMP) could not be evoked (Table 1). Other tests, including complete blood count (CBC), C-reactive protein, coagulation-related tests, immunoglobulin, thyroid-related blood tests, high resolution computed tomography (HRCT) of temporal bone, cranial magnetic resonance imaging (MRI) and chest X-ray reflected no abnormalities. Due to lack of verified low to medium frequency hearing loss in audiometric tests, he was diagnosed with probable MD. During 1 week of treatment with Ginaton, sodium aescinate, and mecobalamin, the boy was discharged without any further attacks of vertigo.

Table 1
| Audiovestibular tests | Dec 2018 | May 2019 | Mar 2023 | |||||
|---|---|---|---|---|---|---|---|---|
| Left (affected) | Right | Left (affected) | Right | Left (affected) | Right | |||
| PTA threshold (0.5, 1, 2 kHz; dB HL) | 13 | 0 | 8 | 2 | 47 | 7 | ||
| SDSmax score, % | 100 | 100 | 100 | 100 | 88 | 96 | ||
| DPOAE | – | – | Pass | Pass | – | – | ||
| ABR (dB nHL) | – | – | 25 | 25 | – | – | ||
| Caloric tests | Left LSC weakness | Left LSC weakness | Normal | |||||
| Unilateral weakness UW, % | 53 | – | 65 | – | 4 (cold) | – | ||
| Directional preponderance DP, % | – | 29 | 39 | −4 (cold) | – | |||
| vHIT gain | ||||||||
| Anterior semicircular canal | 1 | 0.98 | 0.9 | 0.89 | 0.96 | 0.96 | ||
| Horizontal semicircular canal | 1 | 0.92 | 0.78 | 0.77 | 0.97 | 1.01 | ||
| Posterior semicircular canal | 1.01 | 0.84 | 0.75 | 0.83 | 1.01 | 0.99 | ||
| Cervical VEMPs | Not evoked | Normal | Normal | Normal | Normal | Normal | ||
| Ocular VEMPs | Normal | Normal | Normal | Normal | Normal | Normal | ||
| Electrocochleography (SP/AP) | 0.14 | 0.13 | 0.14 | 0.2 | – | – | ||
| Gd-MRI | – | – | – | – | Significant EH | |||
| SOT | ||||||||
| Composite | – | – | 74 | 86 | ||||
| Somatosensory | – | – | 99 | 99 | ||||
| Vision | – | – | 91 | 94 | ||||
| Vestibule | – | – | 63 | 82 | ||||
| Visual preference | – | – | 91 | 105 | ||||
PTA, pure-tone average; SDSmax, speech discrimination score; DPOAE, distortion product otoacoustic emission; ABR, auditory brain stem response; UW, unilateral weakness; DP, directional preponderance; LSC, lateral semicircular canal; vHIT, video head impulse test; VEMP, vestibular evoked myogenic potential; SP/AP, summating the potential/active potential; Gd-MRI, gadolinium-enhanced magnetic resonance imaging; EH, endolymphatic hydrops; SOT, sensory organization test.
He was re-admitted to our hospital 5 months later (14 May, 2019) with more frequent episodes of vertigo which lasted for from 10 minutes to 2 hours and about three times a day. He stated that hearing loss usually occurred after the vertigo stopped whereas tinnitus and aural fullness were consistently present. However, ABR, PTA, and distortion product otoacoustic emission (DPOAE) indicated he had a normal hearing threshold. Caloric tests showed weak response of the left ear (Table 1). The boy was still diagnosed with probable MD due to the lack of objective evidence of hearing loss. Due to the frequent acute attacks of vertigo, we administrated dexamethasone 5 mg intravenously (14 May, 2019) and gradually reduced the dosage to 2.5 mg 2 days later. He was discharged on 21 May in a stable condition. He was advised to take dehydration medication (sodium aescinate) orally and undergo vestibular rehabilitation exercise; he remained free of vertigo during the 1 year of follow-up.
The patient was admitted to Shandong Provincial ENT Hospital due to permanent left-sided aural fullness and tinnitus on 6 March, 2023. PTA showed left-sided moderate sensorineural hearing loss with a speech discrimination score of 88% (Table 1). The results of vestibular exams were within normal limits (Table 1). We did not observe spontaneous nystagmus during his hospitalization. Gadolinium-enhanced MRI (Gd-MRI) indicated significant endolymphatic hydrops (EH) of the left ear (Figure 2). There was nothing abnormal observed in brain MRI and other serological tests. Thus, the boy was finally diagnosed with MD. Methylprednisolone was administered both systemically and postauricularly. Hearing function was obviously improved 5 days later (Figure 1). During our 6-month telephone follow-up, the patient had taken dehydration medication (sodium aescinate) and undergone vestibular rehabilitation exercise regularly; his vertigo and hearing loss have not recurred. He stated that he could attend school and participate in sports activities normally. Timeline of diagnosis and treatment of this patient can be seen in Figure 3. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s legal guardians for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

Discussion
Audiometry might be the only objective standard of MD diagnosis which was mainly based on symptoms and history (5). Tinnitus or aural fullness might initially be linked to episodes of vertigo (5,8). A temporal correlation between hearing loss and the vertigo episode might be not observed by the patient. In this case, hearing loss lagged behind the onset of vertigo episodes by several years. Compared to his previous two admissions, the evaluation of vestibular function such as caloric tests seemed to be normal upon his third admission. Although he was free of vertigo after the second medication, his aural symptoms progressed to permanence without vertiginous attack. One possible explanation is that there might be an early-onset hearing loss, although it is not persistent and there was no evidence of it detected initially. Another possibility is that the boy’s hearing loss did occur in a time gap, independent of vestibular symptoms. What is suggested to us is that the typical symptoms of MD may not appear at the same time initially, so the diagnosis of MD needs to capture evidence over a period of time rather than being limited to a single time point.
Although it is reported that vestibular symptoms and cochlear symptoms might not appear simultaneously in patients with MD, about 1/4 of them only have vertigo symptoms, whereas 1/3 could experience the typical characteristics of vertigo and hearing loss in the early stage (9). It is reported that vestibule and cochlea might be differential sensitivity of cochlear and vestibular sensory cells (10). In addition, the reasons of dissociated symptoms might be different between children and adults. Age-associated changes in balance function can occur during infancy and childhood (6). Vestibular system is not fully developed in children until about 17 years of age (7). In this case, the development of the vestibular system itself, the interaction of vision and proprioception would superimpose the MD-induced impairment of vestibular function. However, the specific effects need to be further studied in more children cases. Importantly, it reminds us of the necessity to prolong the follow-up period of pediatric MD and monitor aural symptoms sensitively even though vertigo did not recur.
Characterized with low frequency vestibular impairment, vestibular tests, such as caloric test and video head impulse test (vHIT) for semicircular canal function and vestibular evoked myogenic potentials (VEMP) for macular function, provide useful auxiliary information. Our patient’s caloric test showed reduced left-sided semicircular function whereas the vHIT reflected normal function. The disassociated results of caloric tests and vHIT are probable and common in the early stages of MD (11). Yilmaz et al. found that 76.7% of patients with MD had disassociated results of abnormal caloric tests but normal vHIT, whereas 50.8% were associated, suggesting that vestibular testing with the caloric test seemed more sensitive for detecting MD (12). As for macular function, this patient could not achieve normal cVEMP but did display normal ocular VEMP (oVEMP) of the affected left ear. It has been reported that the saccule is more susceptible to EH impairment than the utricle (13). It is more likely to find abnormal results in cVEMP than in oVEMP in both adults and children with MD (14).
Although it is not clear whether EH is the cause or the concomitant phenomenon of MD, it is the main pathology change of MD (15,16). Electrocochleography and Gd-MRI can detect EH sensitively. A high SP/AP ratio may therefore be predictive of EH (17,18). Gadolinium (intravenous or local administration) can enter the perilymph and be shown in the delayed MRI, correspondingly reflecting EH (19). In our case, Gd-MRI showed significant EH. Electrocochleography was reported to have a lower sensitivity and a lower negative predictive value than Gd-MRI (17).
Considering the pathological EH, diuretics are still commonly used as a first-line therapy for fluid retention (20). The effectiveness of diuretic therapy in treating vertigo or hearing loss related to MD is not well established and requires a number of critical high-quality randomized control trials (21). Further, as for acute attack of MD, the anti-inflammatory and immunosuppressive properties of corticosteroids, along with their ability to regulate inner ear homeostasis, are the basis for their mechanism in the treatment of MD. Studies have demonstrated that corticosteroids can modulate transcription factors of the nuclear factor κB (NF-κB) family and aquaporins in the inner ear (22,23). Upon the boy’s third admission, we administered corticosteroids postauricularly and systemically due to his profound hearing loss which was then improved obviously. It is reported that systemic alone or postauricular administration of glucocorticoids seem to be equally effective, and the specific effects and mechanisms require further study (24). Since the first report of pediatric MD in 1940, there have been relatively few cases reported in the literature. Meyerhoff documented nine cases of pediatric MD treated with endolymphatic sac drainage procedure to assess its safety and efficacy (25). Nevertheless, the efficacy of this surgery in pediatric patients remains contentious due to developmental and pathophysiological differences compared to adults. Akagi et al. described three pediatric MD cases that achieved prompt recovery with medication (1). Abouzari et al. reported a pediatric MD patient who exhibited complete responsiveness to dietary and lifestyle modifications for migraine, along with magnesium and riboflavin supplementation (26). Another study reported favorable outcomes in a pediatric MD case associated with allergy and dysautonomia following an allergen-restricted diet (3). These studies primarily focus on treatment rather than the progression of MD in children and lack certain modern technical tests to evaluate vestibular function. Although this paper is constrained by a single case report and necessitates further follow-up, it presents a pediatric MD case with dissociated symptoms alongside comprehensive vestibular examinations over a timeline.
Conclusions
Vestibular and cochlear symptoms may occur and progress independently in pediatric MD, and the mechanisms remain to be explored. Audiovestibular evaluation, especially caloric tests and cVEMP, can be useful for diagnosis of pediatric MD. Gd-MRI can be sensitive for detecting EH. Corticosteroids therapy might be effective in alleviating acute attacks of vertigo and hearing loss. It is meaningful to prolong the follow-up period of pediatric patients with MD.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-23/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-23/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-23/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s legal guardians for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akagi H, Yuen K, Maeda Y, et al. Ménière's disease in childhood. Int J Pediatr Otorhinolaryngol 2001;61:259-64. [Crossref] [PubMed]
- Hausler R, Toupet M, Guidetti G, et al. Menière's disease in children. Am J Otolaryngol 1987;8:187-93. [Crossref] [PubMed]
- Meyerhoff WL, Paparella MM, Shea D. Ménière's disease in children. Laryngoscope 1978;88:1504-11. [Crossref] [PubMed]
- Casani AP, Dallan I, Navari E, et al. Vertigo in childhood: proposal for a diagnostic algorithm based upon clinical experience. Acta Otorhinolaryngol Ital 2015;35:180-5.
- Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Menière's disease. J Vestib Res 2015;25:1-7. [Crossref] [PubMed]
- Ferber-Viart C, Ionescu E, Morlet T, et al. Balance in healthy individuals assessed with Equitest: maturation and normative data for children and young adults. Int J Pediatr Otorhinolaryngol 2007;71:1041-6. [Crossref] [PubMed]
- Sinno S, Dumas G, Mallinson A, et al. Changes in the Sensory Weighting Strategies in Balance Control Throughout Maturation in Children. J Am Acad Audiol 2021;32:122-36. [Crossref] [PubMed]
- Phillips J, Murdin L, Khondoker M, et al. Cluster Analysis to Identify Clinical Subtypes of Ménière's Disease. Laryngoscope. 2024; Epub ahead of print. [Crossref]
- Harcourt J, Barraclough K, Bronstein AM. Meniere's disease. BMJ 2014;349:g6544. [Crossref] [PubMed]
- Hara M, Kimura RS. Morphology of the membrana limitans. Ann Otol Rhinol Laryngol 1993;102:625-30. [Crossref] [PubMed]
- Lee JY, Kwon E, Kim HJ, et al. Dissociated Results between Caloric and Video Head Impulse Tests in Dizziness: Prevalence, Pattern, Lesion Location, and Etiology. J Clin Neurol 2020;16:277-84. [Crossref] [PubMed]
- Yilmaz MS, Egilmez OK, Kara A, et al. Comparison of the results of caloric and video head impulse tests in patients with Meniere's disease and vestibular migraine. Eur Arch Otorhinolaryngol 2021;278:1829-34. [Crossref] [PubMed]
- Okuno T, Sando I. Localization, frequency, and severity of endolymphatic hydrops and the pathology of the labyrinthine membrane in Menière's disease. Ann Otol Rhinol Laryngol 1987;96:438-45. [Crossref] [PubMed]
- Wang C, Wu CH, Cheng PW, et al. Pediatric Meniere's disease. Int J Pediatr Otorhinolaryngol 2018;105:16-9. [Crossref] [PubMed]
- Mohseni-Dargah M, Falahati Z, Pastras C, et al. Meniere's disease: Pathogenesis, treatments, and emerging approaches for an idiopathic bioenvironmental disorder. Environ Res 2023;238:116972. [Crossref] [PubMed]
- Xu W, Li X, Song Y, et al. Ménière's disease and allergy: Epidemiology, pathogenesis, and therapy. Clin Exp Med 2023;23:3361-71. [Crossref] [PubMed]
- Ziylan F, Smeeing DP, Stegeman I, et al. Click Stimulus Electrocochleography Versus MRI With Intratympanic Contrast in Ménière's Disease: A Systematic Review. Otol Neurotol 2016;37:421-7. [Crossref] [PubMed]
- Noh TS, Park MK, Lee JH, et al. Endolymphatic hydrops asymmetry distinguishes patients with Meniere's disease from normal controls with high sensitivity and specificity. Front Neurol 2023;14:1280616. [Crossref] [PubMed]
- Bernaerts A, Vanspauwen R, Blaivie C, et al. The value of four stage vestibular hydrops grading and asymmetric perilymphatic enhancement in the diagnosis of Menière's disease on MRI. Neuroradiology 2019;61:421-9. [Crossref] [PubMed]
- Basura GJ, Adams ME, Monfared A, et al. Clinical Practice Guideline: Ménière's Disease Executive Summary. Otolaryngol Head Neck Surg 2020;162:415-34. [Crossref] [PubMed]
- Stern Shavit S, Lalwani AK. Are diuretics useful in the treatment of meniere disease? Laryngoscope 2019;129:2206-7. [Crossref] [PubMed]
- Dong SH, Kim SS, Kim SH, et al. Expression of aquaporins in inner ear disease. Laryngoscope 2020;130:1532-9. [Crossref] [PubMed]
- Nevoux J, Viengchareun S, Lema I, et al. Glucocorticoids stimulate endolymphatic water reabsorption in inner ear through aquaporin 3 regulation. Pflugers Arch 2015;467:1931-43. [Crossref] [PubMed]
- Chen D, Li Z, Zhou Q, et al. Impacts of different methylprednisolone administration routes in patients with sudden hearing loss or Meniere's disease. J Otol 2020;15:149-54. [Crossref] [PubMed]
- Wu H, Gao Z. Vertigo with dysautonomia and serious allergy: An unusual case of juvenile Ménière's disease. Int J Pediatr Otorhinolaryngol 2015;79:2438-41. [Crossref] [PubMed]
- Abouzari M, Abiri A, Djalilian HR. Successful treatment of a child with definite Meniere's disease with the migraine regimen. Am J Otolaryngol 2019;40:440-2. [Crossref] [PubMed]


