
Characterization of brain development with neuroimaging in a female mouse model of chemotherapy treatment of acute lymphoblastic leukemia
Highlight box
Key findings
• Brain developmental patterns were different in mice treated with intrathecal methotrexate and oral dexamethasone, relative to saline-treated controls.
What is known and what is new?
• Cross-sectional data suggest that long-term survivors of childhood leukemia exhibit abnormal brain morphology, which has been corroborated by ex vivo neuroimaging studies in mice.
• The present study adds knowledge on longitudinal brain growth following chemotherapy exposures.
What is the implication, and what should change now?
• Preclinical neuroimaging data provide novel information on long-term neurotoxicity of chemotherapy treatment for pediatric leukemia.
Introduction
Survival rates for childhood acute lymphoblastic leukemia (ALL) exceed 90% on current treatment protocols involving administration of risk-stratified intrathecal methotrexate (IT-MTX), dexamethasone (DEX), and other agents (1). Although treatment-associated neurotoxicity has improved in recent decades, childhood ALL survivors remain vulnerable to neurocognitive impairments that affect quality of life (2,3). Previous work in childhood ALL survivors treated with chemotherapy alone have observed associations between neurocognitive impairment and cumulative exposures to IT-MTX and DEX (4-6). Neuroimaging studies demonstrate widespread reductions in gray matter (GM) and white matter (WM), including the nucleus accumbens, amygdala, cerebellum, thalamus, hippocampus, and corpus callosum (7-9). Plasma MTX concentrations have also been found to be associated with functional brain activity, WM integrity in the frontostriatal tract, and cortical thickness in the dorsolateral prefrontal regions (10). Additionally, survivors who received a greater number of IT-MTX injections exhibited less efficient neural network connectivity relative to survivors who received fewer IT injections (11). Improved understanding of chemotherapy-associated neurotoxicity and progression over time is essential in mitigating risk of neurocognitive impairment. Patients undergoing chemotherapy receive multiple agents through various modalities which makes it challenging to identify specific mechanisms of neurotoxicity (1). While clinical neuroimaging studies have been instrumental in understanding neurocognitive late effects following ALL therapy, these studies are confounded by uncontrolled patient-related factors such as age at diagnosis, treatment intensity, and other psychosocial attributes. Animal models afford opportunities to test hypotheses regarding the impact of chemotherapy on brain development in a controlled environment, within genetically homogenous species (12).
The majority of animal studies have explored the effects of chemotherapy agents using cell biology (13,14), and have provided evidence that chemotherapy agents such as MTX interfere with neurogenesis, gliogenesis, and myelin fiber integrity. Further, DEX has been associated with the upregulation of pro-inflammatory cytokines and gliosis, particularly in the hippocampus (15). While these studies have provided invaluable information about the mechanisms of chemotherapy-induced neurotoxicity, there are limitations to the generalizability of results obtained from cultured cells.
In vivo neuroimaging in animal models enables repeatable, whole-brain measurements to characterize brain growth trajectories across the lifespan, making it an attractive method to study the long-term impact of chemotherapy treatment on the developing brain (1). Spencer Noakes and colleagues (12) utilized longitudinal neuroimaging to demonstrate that exposure to vincristine in young mice was associated with significant alterations in structural brain growth in several different regions at the human age equivalent of early adulthood (12). Using ex vivo magnetic resonance imaging (MRI), the authors also demonstrated that intra-orbitally administered methotrexate and DEX resulted in neuroanatomical changes relative to saline-treated mice (12). Likewise, Berlin and colleagues used ex vivo diffusion tensor imaging to quantify WM in rats that received MTX via intraperitoneal injection for four weeks. Compared to controls, rats exposed to MTX exhibited lower fractional anisotropy across major WM bundles (16).
However, several knowledge gaps remain to be addressed. First, longitudinal patterns of brain growth following IT-MTX and DEX have not been investigated in preclinical models, even though these agents have been associated with alterations in brain structure and function in cross-sectional clinical studies (5,6). Second, animal studies typically report on the effects of isolated chemotherapy agents, even though pediatric ALL patients receive combined agents throughout therapy (17). Third, while mode of chemotherapy delivery is an important factor of ALL therapy, preclinical studies typically do not account for method of drug delivery (e.g., CNS-directed or oral administration) which could be associated with differential neurotoxicity (12). The present study sought to take the first step in addressing these issues by determining the feasibility of utilizing in vivo neuroimaging to characterize longitudinal brain changes in mice who received IT-MTX and oral DEX. We present this article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-458/rc).
Methods
A protocol was prepared before the study without registration. Healthy, treatment-naïve C57BL/6 wild-type mice obtained from Jackson Laboratory (Bar Harbor, ME) were used in this study. Mice were housed in groups of 5 and nesting materials were provided as enrichment. Littermates were used to minimize genetic and environmental variability among cage mates. Both treatment and control mice were provided DietGel® Recovery (ClearH2O, INC., Westbrook, ME) during the injection period in additional to suspended food pellets to ensure proper nutrition. All procedures were done in the Animal Resource Center at St. Jude Children’s Research Hospital (Memphis, TN). Injections were performed by trained veterinarian technicians in designated procedure rooms. Experiments were performed under an approved project (ARC #: 652-100632) granted by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee, in compliance with national guidelines detailed in the Animal Welfare Act (PHS Assurance ID: D16-00043 [A3077-01]).
Chemotherapy protocol
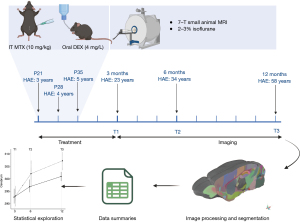
A summary of the study methods is visualized in Figure 1. Twenty female juvenile mice were randomly assigned to receive chemotherapy and DEX (n=10; IT-MTX + DEX) or saline only (n=10; randomization and experimental procedures performed by T.A.). Following an established mouse model of intrathecal ALL therapy that mimics current childhood ALL treatment (18), intrathecal injections were administered between the L5 and L6 vertebrae at 21 days old [human age equivalent (HAE) =3 years], 28 days old (HAE =4 years), and 35 days old (HAE =5 years) under general anesthesia with 3–4% isoflurane. Pharmaceutical-grade, preservative-free MTX injection USP from Teva Pharmaceuticals. MTX or saline was slowly injected into the spinal column using pre-filled syringes with 29-gauge needles, with a total volume administration of 5–10 µL depending on body weight. The animals were subsequently transferred to a recovery cage for close monitoring before returning to their home cage. The IT-MTX + DEX group received 10 mg/kg MTX, and the control group received saline injections of equivalent volume. Mice in the IT-MTX + DEX group were immediately given oral DEX (4 mg/L) in their drinking water for four weeks; equivalent to 1.33 mg/kg/day according to established mouse models of oral DEX (19). All mice were given antibiotics in their drinking water (e.g., tetracycline: 1 g/L; sulfamethoxazole: 600 mg/L; trimethoprim: 102 mg/L) to prevent infections from the DEX-induced immune suppression.
Neuroimaging
Neuroimaging was completed using a 7T Bruker ClinScan small animal MRI system (Bruker Biospin MRI GmbH, Ettlingen, Germany) at the Center for In Vivo Imaging and Therapeutics, which is equipped with an integrated animal handling system with positioner, heating system, anesthesia, and respiratory monitoring apparatus. Temperature, respiration, and heart rate were monitored throughout the scanning session. The system was equipped with four digital receiver channels allowing the operation of multi-channel coils to enhance signaling. Images were acquired with a 4-channel radio frequency (RF) receive-only surface coil placed directly on top of the head and placed inside the magnet within a circularly polarized (CP) whole body RF coil. Mice were sedated with 2-3% isoflurane administered via a nose cone. The neuroimaging acquisition took approximately 10 minutes to complete. All mice were scanned at 3 (T1), 6 (T2), and 12 months (T3) post-treatment (approximate HAE = 23, 34, and 58 years, respectively) (20). At each timepoint, multiple, anisotropic T2-weighted (T2w) images were each acquired in a different acquisition plane specified in Table S1.
Each multiplanar acquisition was first reoriented to a consistent right-to-left, posterior-to-anterior, inferior-to-superior (RPI) orientation before being rigidly aligned to a mouse brain template (21) and resampled to 70 micron isotropic resolution using a cubic spline interpolation. An initial brain mask was translated to each image by calculating an affine registration to the atlas template. Each acquisition was then denoised, intensity inhomogeneity was corrected, and voxel intensities were rescaled setting the maximum in-brain value to 3,000. Multiplanar images were combined using an iterative template building procedure, where acquisitions were averaged and co-registered iteratively. Using the average T2w image per mouse, the same iterative template building procedure was used to construct a study-specific template. The study-specific template was co-registered using a rigid, affine, and symmetric normalization procedure to the standard atlas. Each mouse underwent a final high-fidelity, non-linear registration to the study template. Transformations were applied in a single interpolation. Jacobian determinants were calculated for the deformation matrix, excluding rigid components, to visualize voxel-wise transformations. A priori brain regions were selected based on the literature, and included: total GM, total WM, cerebrum, cerebellum, corpus callosum, claustrum, amygdala, striatum, thalamus, hypothalamus, hippocampus, and amygdala. These procedures were conducted in a blinded fashion to treatment status of mice.
Statistical analysis
Mice that were alive for at least one scanning timepoint were included in the analyses. Raw data were plotted for each observation to inspect distribution. For each region-of-interest (ROI), change in brain volume was calculated between three consecutive timepoints. The differences in the time intervals between scans (3-, 6-, and 12 months) was accounted for by calculating the volume change and dividing the value by the duration between scans. Volume change was assessed at age 6 months (i.e., T1 to T2) and at 12 months (i.e., T2 to T3). Linear models included ROI volume change as dependent variables and group (i.e., saline vs. IT-MTX + DEX) as the independent variable. Analyses were conducted in R version 4.2.1, and statistical tests were two-sided.
Results
Of the 20 mice included in the study, five mice in the IT-MTX + DEX group died prior to scanning as a result of the injection procedure. Three mice in the saline group died prior to the final scan.
Figure 2 visualizes the findings across ROIs, and Tables 1,2 list the group estimates for volume change at 6 months (T1 to T2) and at 12 months (T2 to T3), respectively. Regional volume change between T1 and T2 was significantly different between groups for total GM [estimate =2.06, 95% confidence interval (CI): 0.27–3.84], the cerebrum (estimate =1.62, 95% CI: 0.14–3.09), claustrum (estimate =0.06, 95% CI: 0.02–0.09), amygdala (estimate =0.16, 95% CI: 0.03–0.29), and striatum (estimate =0.18, 95% CI: 0.01–0.35). For each of these regions, the IT-MTX + DEX group exhibited more robust growth than the saline group. As evidenced by the positive estimates in Table 1, this pattern was consistent across all ROIs.
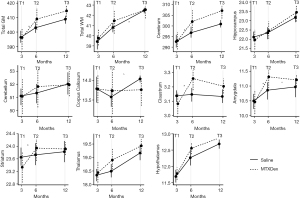
Table 1
| ROI | Group estimate | 95% CI | t(df) | P |
|---|---|---|---|---|
| Total GM | 2.06 | 0.27 to 3.84 | t(13)=2.49 | 0.03 |
| Total WM | 0.10 | −0.34 to 0.54 | t(13)=0.49 | 0.63 |
| Cerebrum | 1.62 | 0.14 to 3.09 | t(13)=2.36 | 0.03 |
| Cerebellum | 0.18 | −0.03 to 0.38 | t(13)=1.89 | 0.08 |
| Corpus Callosum | 0.06 | −0.23 to 0.35 | t(13)=0.42 | 0.68 |
| Claustrum | 0.06 | 0.02 to 0.09 | t(13)=3.08 | 0.009 |
| Striatum | 0.18 | 0.01 to 0.35 | t(13)=2.25 | 0.04 |
| Thalamus | 0.11 | −0.04 to 0.25 | t(13)=1.59 | 0.14 |
| Hypothalamus | 0.07 | −0.06 to 0.20 | t(13)=1.13 | 0.28 |
| Hippocampus | 0.08 | −0.12 to 0.29 | t(13)=0.90 | 0.39 |
| Amygdala | 0.16 | 0.03 to 0.29 | t(13)=2.74 | 0.02 |
Bold values highlight P<0.05. ROI, region of interest; GM, gray matter; WM, white matter; CI, confidence interval.
Table 2
| Variable | Group estimate | 95% CI | t(df) | P |
|---|---|---|---|---|
| Total GM | −0.08 | −0.97 to 0.82 | t(10)=−0.19 | 0.85 |
| Total WM | −0.14 | −0.27 to −0.01 | t(10)=−2.38 | 0.04 |
| Cerebrum | 0.06 | −0.66 to 0.78 | t(10)=0.19 | 0.86 |
| Cerebellum | −0.10 | −0.35 to 0.15 | t(10)=−0.89 | 0.40 |
| Corpus Callosum | −0.09 | −0.19 to 0.00 | t(10)=−2.20 | 0.05 |
| Claustrum | −0.01 | −0.03 to 0.02 | t(10)=−0.56 | 0.59 |
| Striatum | −0.03 | −0.11 to 0.05 | t(10)=−0.76 | 0.47 |
| Thalamus | −0.02 | −0.13 to 0.08 | t(10)=−0.47 | 0.65 |
| Hypothalamus | −0.02 | −0.08 to 0.05 | t(10)=−0.56 | 0.59 |
| Hippocampus | 0.04 | −0.01 to 0.09 | t(10)=1.77 | 0.11 |
| Amygdala | −0.05 | −0.10 to 0.00 | t(10)=−2.21 | 0.05 |
Bold value highlights P<0.05 and italicized values highlights P=0.05 when rounded (the values are 0.052 and 0.051). ROI, region of interest; GM, gray matter; WM, white matter; CI, confidence interval.
Volumes continued to increase between T2 and T3 across groups; however, growth was significantly less robust in the IT-MTX + DEX compared to the saline group for total WM (estimate =−0.14, 95% CI: −0.27 to −0.01). Likewise, growth in the corpus callosum (estimate =−0.09, 95% CI: −0.19 to 0.00) and amygdala (estimate =−0.05, 95% CI: −0.10 to 0.00) was attenuated in the IT-MTX + DEX group compared with the saline group. Group estimates were mostly negative between T2 and T3, suggesting a similar patterns across ROIs.
Discussion
This study demonstrated proof of concept for assessing the long-term effects of IT-MTX and oral DEX with neuroimaging in a mouse model of childhood ALL therapy. A systematic review of the neuroimaging literature in pediatric ALL survivors treated with chemotherapy reported that the majority of studies have been cross-sectional and conducted during adolescence or young adulthood (7). Neurodevelopment is a dynamic process with continuous change (22,23). Therefore, it is essential to evaluate trajectories of change across the lifespan to better characterize brain development following chemotherapy. However, longitudinal studies in humans can be costly, time consuming, and challenging due to attrition and uncontrollable human factors that may impact research outcomes. While caution is warranted when comparing outcomes between species, supplementing human observations with experiments in mice is a critical step to elucidate mechanisms of treatment-related neurocognitive late effects. Pending replication, our results imply that exposure to IT-MTX + DEX during early development may be associated with alterations in brain developmental trajectories throughout the lifespan.
While others have investigated the impact of isolated chemotherapy agents on the mouse brain using neuroimaging (12), longitudinal brain patterns have not been reported for MTX and DEX. In the present study, we demonstrated that IT-MTX + DEX exposed mice initially exhibited more robust growth in regional brain volume relative to saline-treated mice. As demonstrated by literature in children with autism spectrum disorder (24), increased growth rate, or hyper-expansion, does not necessarily reflect improved brain function. Mechanisms of hyper-expansion remain to be fully elucidated, particularly in the context of chemotherapy exposures. However, reactive gliosis following insults to the CNS is known to trigger proliferation and hypertrophy of various glial cells (25). Moreover, repeated exposure to CNS-directed MTX can disrupt reactive glial function and inhibit neuroplasticity (25,26). Interestingly, intraperitoneally injected DEX did not ameliorate gliosis in a mouse model of neurodegeneration (27).
The initial period of potential hyper-expansion was followed by attenuated growth in the chemotherapy-exposed group, particularly in WM. The mouse brain continues to increase in volume throughout late adulthood (28); thus, the observed attenuation of growth later in life in the chemotherapy-exposed mice may reflect accelerated aging (29). Prevalence of frailty among young adult childhood cancer survivors is similar to that of aging adults (30), and it has been hypothesized that childhood cancer and its treatment may cause a shift in the normal aging trajectory (29). MTX and DEX have been demonstrated to cause neuronal and glial injury (26), and the CNS may initially attempt to repair and/or cope with the neurotoxic effects of these agents. However, these coping mechanisms may become less effective with increased age. Consistent with the notion of neural coping, fMRI results in childhood ALL survivors have indicated that the brain may require additional resources to support neurocognitive functions (31). Our knowledge of longitudinal brain changes in aging childhood cancer survivors is limited. At minimum, the results from the current study may help generate hypotheses about aging trajectories in survivors.
Certain limitations of the study warrant consideration. First, the sample included few mice and replication is required prior to making firm conclusions about changes in brain growth in response to IT-MTX + DEX. Second, behavioral data were not collected and as such, the functional consequences of the observed patterns of growth were not evaluated. A behavioral study in rats demonstrated that combined therapies—encompassing cranial radiation, MTX, and prednisone—caused behavioral deficits. Moreover, prednisone appeared to enhance the adverse impact of MTX and radiation (32). More recent work by Wen and colleagues showed that longitudinal exposures to systemic and IT MTX resulted in persistent cognitive deficits in rats (33). Third, while experiments were designed to mimic the clinical experience, treatment exposures in mice were compressed into a timeframe spanning a few weeks. The short lifespan of mice necessitates time compression and affords opportunities for efficient longitudinal assessment; however, the experimental protocol does not fully reflect the extent, intensity, and phasing of ALL therapy. Relatedly, patients with ALL require a variety of agents beyond MTX and DEX, some of which have been shown to have neurotoxic properties (e.g., vincristine) (12). Fourth, observations were limited to female mice, hence, our results are not necessarily generalizable to males. As shown in a growing number of clinical (2,34) and animal studies (33), sex is an important consideration when evaluating neurocognitive development in the context of chemotherapy exposures.
Conclusions
The present study illustrates the potential benefit of leveraging neuroimaging in mouse models of cancer therapy. The results imply that IT-MTX + DEX chemotherapy exposures affect longitudinal brain developmental changes. Our knowledge of chemotherapy-associated brain changes remains limited, and transdisciplinary efforts are required to gain insight into strategies to prevent and/or mitigate neurocognitive late effects associated with pediatric ALL.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-458/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-458/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-458/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-458/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under an approved project (ARC #: 652-100632) granted by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee, in compliance with national guidelines detailed in the Animal Welfare Act (PHS Assurance ID: D16-00043 [A3077-01]).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011;29:551-65. [Crossref] [PubMed]
- van der Plas E, Qiu W, Nieman BJ, et al. Sex-Specific Associations Between Chemotherapy, Chronic Conditions, and Neurocognitive Impairment in Acute Lymphoblastic Leukemia Survivors: A Report From the Childhood Cancer Survivor Study. J Natl Cancer Inst 2021;113:588-96. [Crossref] [PubMed]
- Kunin-Batson A, Kadan-Lottick N, Neglia JP. The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia. Psychooncology 2014;23:692-9. [Crossref] [PubMed]
- van der Plas E, Modi AJ, Li CK, et al. Cognitive Impairment in Survivors of Pediatric Acute Lymphoblastic Leukemia Treated With Chemotherapy Only. J Clin Oncol 2021;39:1705-17. [Crossref] [PubMed]
- Edelmann MN, Ogg RJ, Scoggins MA, et al. Dexamethasone exposure and memory function in adult survivors of childhood acute lymphoblastic leukemia: A report from the SJLIFE cohort. Pediatr Blood Cancer 2013;60:1778-84. [Crossref] [PubMed]
- Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol 2013;31:4407-15. [Crossref] [PubMed]
- Gandy K, Scoggins MA, Jacola LM, et al. Structural and Functional Brain Imaging in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated With Chemotherapy: A Systematic Review. JNCI Cancer Spectr 2021;5:pkab069. [Crossref] [PubMed]
- van der Plas E, Schachar RJ, Hitzler J, et al. Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain Behav 2017;7:e00621. [Crossref] [PubMed]
- van der Plas E, Spencer Noakes TL, Butcher DT, et al. Quantitative MRI outcomes in child and adolescent leukemia survivors: Evidence for global alterations in gray and white matter. Neuroimage Clin 2020;28:102428. [Crossref] [PubMed]
- Krull KR, Cheung YT, Liu W, et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34:2644-53. [Crossref] [PubMed]
- Kesler SR, Ogg R, Reddick WE, et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain Connect 2018;8:333-42. [Crossref] [PubMed]
- Spencer Noakes TL, Przybycien TS, Forwell A, et al. Brain Development and Heart Function after Systemic Single-Agent Chemotherapy in a Mouse Model of Childhood Leukemia Treatment. Clin Cancer Res 2018;24:6040-52. [Crossref] [PubMed]
- Matsos A, Johnston IN. Chemotherapy-induced cognitive impairments: A systematic review of the animal literature. Neurosci Biobehav Rev 2019;102:382-99. [Crossref] [PubMed]
- Yamamura M, Hanamura K, Koganezawa N, et al. Impacts of methotrexate on survival, dendrite development, and synapse formation of cortical neurons. Genes Cells 2023;28:563-72. [Crossref] [PubMed]
- Fardell JE, Zhang J, De Souza R, et al. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology (Berl) 2014;231:841-52. [Crossref] [PubMed]
- Berlin C, Lange K, Lekaye HC, et al. Long-term clinically relevant rodent model of methotrexate-induced cognitive impairment. Neuro Oncol 2020;22:1126-37. [Crossref] [PubMed]
- Ossorio-Salazar VA, D'Hooge R. Methodological shortcomings of preclinical research on chemotherapy-induced cognitive impairment. Neurosci Biobehav Rev 2023;150:105198. [Crossref] [PubMed]
- Alexander TC, Simecka CM, Kiffer F, et al. Changes in cognition and dendritic complexity following intrathecal methotrexate and cytarabine treatment in a juvenile murine model. Behav Brain Res 2018;346:21-8. [Crossref] [PubMed]
- Sze CI, Lin YC, Lin YJ, et al. The role of glucocorticoid receptors in dexamethasone-induced apoptosis of neuroprogenitor cells in the hippocampus of rat pups. Mediators Inflamm 2013;2013:628094. [Crossref] [PubMed]
- Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci 2016;152:244-8. [Crossref] [PubMed]
- Dorr AE, Lerch JP, Spring S, et al. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008;42:60-9. [Crossref] [PubMed]
- Bethlehem RAI, Seidlitz J, White SR, et al. Brain charts for the human lifespan. Nature 2022;604:525-33. [Crossref] [PubMed]
- Semple BD, Blomgren K, Gimlin K, et al. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013;106-107:1-16. [Crossref] [PubMed]
- Piven J, Arndt S, Bailey J, et al. Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 1996;35:530-6. [Crossref] [PubMed]
- Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 2016;1862:483-91. [Crossref] [PubMed]
- Gibson EM, Nagaraja S, Ocampo A, et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 2019;176:43-55.e13. [Crossref] [PubMed]
- Ye X, Zou G, Hou J, et al. Dexamethasone does not ameliorate gliosis in a mouse model of neurodegenerative disease. Biochem Biophys Rep 2020;24:100817. [Crossref] [PubMed]
- Maheswaran S, Barjat H, Rueckert D, Bate ST, Howlett DR, Tilling L, et al. Longitudinal regional brain volume changes quantified in normal aging and Alzheimer's APP× PS1 mice using MRI. Brain research. 2009;1270:19-32. [Crossref] [PubMed]
- Schuitema I, Alexander T, Hudson MM, et al. Aging in Adult Survivors of Childhood Cancer: Implications for Future Care. J Clin Oncol 2021;39:1741-51. [Crossref] [PubMed]
- Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 2013;31:4496-503. [Crossref] [PubMed]
- Gandy K, Sapkota Y, Scoggins MA, et al. Genetic variants, neurocognitive outcomes, and functional neuroimaging in survivors of childhood acute lymphoblastic leukemia. JNCI Cancer Spectr 2023;7:pkad039. [Crossref] [PubMed]
- Mullenix PJ, Kernan WJ, Schunior A, et al. Interactions of steroid, methotrexate, and radiation determine neurotoxicity in an animal model to study therapy for childhood leukemia. Pediatr Res 1994;35:171-8. [Crossref] [PubMed]
- Wen J, Patel C, Diglio F, et al. Cognitive impairment persists at least 1 year after juvenile rats are treated with methotrexate. Neuropharmacology 2022;206:108939. [Crossref] [PubMed]
- Gandy K, Scoggins MA, Phillips N, et al. Sex-Based Differences in Functional Brain Activity During Working Memory in Survivors of Pediatric Acute Lymphoblastic Leukemia. JNCI Cancer Spectr 2022;6:pkac026. [Crossref] [PubMed]


