
Minimal invasive approaches for pediatric & congenital heart surgery: safe, reproducible, more cosmetic than through sternotomy, and here to stay
Introduction
Surgical repair for the most common congenital heart defects, both in the pediatric and young adult population, has achieved a level of excellence pertaining to safety and quality, thereby setting benchmarks which are internationally reproducible. These defects include an atrial septal defect (ASD), with or without partial anomalous pulmonary venous return (PAPVR), a ventricular septal defect (VSD), partial atrio-ventricular canal (pAVC) with mitral valve cleft, double chambered right ventricle (DCRV), cor triatriatum and scimitar syndrome, whose surgical repairs are expected to be performed with a near 100% success and survival rate, minimal morbidity, and few if any need for reoperations owing to residual or recurring defects (1). The path towards achieving such excellent outcomes is the result of decades of protocol, practice, performance, and teaching of reproducible surgical techniques, enhancement of cardiopulmonary bypass technology, intra-operative anesthesia management, and post-operative intensive care protocols, thus establishing the gold standards we expect today from open heart surgery through a median sternotomy.
High expectations have appropriately been set, first and foremost with regards to surviving an operation (no mortality), but also pertaining to minimizing morbidity. Although partly subjective (comfort, mobilization, functional recovery, pain control), minimizing morbidity can objectively be quantified, including but not limited to, reducing the length of stay in the intensive care unit or hospital, eliminating residual lesions which leave hemodynamic burdens on the heart or even lead to unplanned reoperations, and avoiding true complications which may lead to reduced functional capacity owing to nerve injury (block and pacemaker implantation, phrenic injury and diaphragmatic plication, or recurrent laryngeal injury and hoarseness with difficulty swallowing). While many centers now implement this level of care on a routine basis at all levels, with surgeons using the traditional median sternotomy as their approach to the heart, there is a paradigm shift swinging towards alternative and less invasive ways to achieve the same excellent outcomes.
Since 100% operative survival is now an expected outcome, the paradigm shift is swinging towards obtaining more than just life, but rather striving for enhanced quality of life. In this spirit, many surgical disciplines, including cardiac surgery, are adopting minimal invasive approaches (2-24). In patients (children) with few or no symptoms, elective surgery for the repair of a heart defect may not take on the aspect of a life-saving procedure, but one which is recommended at some point. As such, minimal invasive approaches, while achieving the same excellent cardiac repair results as more invasive traditional methods, aspire to be less disruptive to lifestyle, with shorter in-hospital length of stay, accelerated return to functionality using a muscle-sparing approach, accelerated return to siblings and family, much improved cosmesis with a hidden scar, and thereby an enhanced return to quality of life (2,4,9,14,18-24).
The most obvious landmark after traditional cardiac surgery is the midline median sternotomy incision, used both in children and adults, as the standard approach to perform open heart surgery. The minimal invasive approach aims to not only replace the large visible scar with something more discretely “tucked away”, thereby enhancing quality of life through obviously improved cosmesis, but also by many other lesser mentioned goals of the minimal invasive school of thought, namely through accelerated recovery and return to physical functionality, and by losing the stigma of being a “heart patient”, with its potential positive psychological implications (2,4), especially in growing children. These goals and results will be presented individually, through technical explanations, and whose results are based on an 18-year and ongoing experience spanning multiple institutions, with over 320 patients (2,4,18-21,25,26).
Incision
While different minimally invasive incisions have been described, including the lower mini-sternotomy, the right sub-mammary thoracotomy, the horizontal right axillary thoracotomy, and the anterolateral mini-thoracotomy (5,6,8,11-17), we will primarily focus on the vertical mini right axillary thoracotomy, which has been the mainstay incision of the senior author (Dodge-Khatami A) for nearly two decades (2,4,18-21,25,26). Compared to other incisions, advantages of the vertical mini right axillary thoracotomy include being far from breast tissue with reduced risk of asymmetrical breast growth (2-4), being muscle sparing with the exception of a few fibers of the serratus anterior, allowing rapid functional recovery of the right shoulder and arm, and superior cosmetic results with an incision fully tucked away under the right axilla, which is practically invisible in a standing child/patient with arms rested by the side (Figures 1,2). The details and illustrations of the incision are available in a prior publication by our group, as well as online in surgical videos (2,4,18-21,25,26). Briefly, the patient is positioned in a left lateral decubitus, and the approach involves a muscle-sparing incision made high under the right axilla in the mid-axillary line. The incision corresponds exactly to the anterior border of the latissimus dorsi muscle (Figure 3). The right groin vessels are always prepped and draped in case peripheral cannulation becomes necessary for cardio-pulmonary bypass. The third or fourth rib is exposed after dividing some fibers of the serratus muscle parallel to the rib, the periosteum peeled off the rib and lifted, and after asking the anesthesiologist to discontinue positive end-expiratory pressure (PEEP; which remains off until reapproximating the ribs at the end of the operation), the respective intercostal space is entered through the periosteal cuff (Figure 4). This detail becomes important at chest closure to respect the intercostal space and avoid rib fusion.


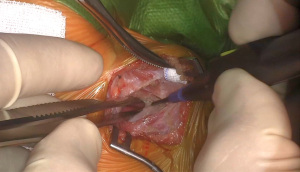
The pericardium is incised 1–2 cm anteriorly to the phrenic nerve and opened longitudinally, keeping the lung behind traction sutures, thereby exposing the right atrium, the SVC, the aortic root, and right ventricular shoulder.
Cannulation strategies
In our experience, the vertical mini right axillary thoracotomy has been performed from the lowest weight of 4.5 kg, up until adult weights beyond 105 kg. Between the weights of 4.5 kg to approximately 40 kg, the operation is actually most similar and straightforward compared to access through a median sternotomy. As such, it may be done with standard micro surgical instruments, as the heart is quite close to the chest wall, with preference for central cannulation for cardiopulmonary bypass (aorto-bicaval). After intravenous heparin, before ascending aortic cannulation, a stay suture is placed on the right atrial appendage, which is retracted caudally, giving better exposure to the ascending aorta. The superior caval vein (SVC) is cannulated with an angled cannula, partial bypass commenced, followed by inferior caval vein (IVC) cannulation and establishment of full bypass. In larger patients, teenagers and young adults around 50 kg or more, consideration may be given to using surgical instruments specifically designed for minimal invasive surgery or thoracoscopy, when available. The aorta is quite remote from the chest wall, and may either be cannulated directly (central cannulation), with or without the use of cannulae permitting the Seldinger technique, or alternatively, right femoral or iliac artery cannulation may be preferred (peripheral cannulation, either directly or into an appropriately sized side Goretex graft sutured to the vessel). Similarly, if direct cannulation is deemed cumbersome or difficult, pre-operative judicious use of a cannula using sterile technique and inserted percutaneously by anesthesia in the right internal jugular vein and advanced in the SVC, and/or peripheral cannulation of the right femoral/iliac vein and advanced up into the IVC, may free up the surgical field, and allow more precise and expeditious intracardiac repair.
Myocardial protection/bypass
After commencing partial and then full cardiopulmonary bypass, regardless of central or peripheral cannulation, most defects are amenable to safe and complete repair at near normothermia or moderate hypothermia between 32–35 degrees Celsius, at full flow. We recommend use of a left vent, most often inserted through the right upper pulmonary vein, or occasionally directly through an ASD, for decompression of the left heart and optimization of a dry surgical field, so reduced bypass flows can be avoided.
Myocardial protection may be selected according to the ease of placing a cardioplegia needle in the ascending aorta and safely being able to cross-clamp the aorta, or not, and the expected duration of intracardiac repair. As such, alternative strategies to cardioplegia and clamping such as induced ventricular fibrillation (VF) are necessary, and the appropriate equipment should always be at hand.
In the vast majority of our cases, aortic cross-clamping and antegrade cardioplegia with a del Nido solution has been used, which even at near normothermia allows for safe ischemic times of approximately 60 minutes. As such, after administering a dose of 30–40 mL/kg, the cardioplegia needle, which itself is space-occupying and cumbersome, may be removed, and the intracardiac repair performed. Even after appropriate and extensive deairing measures using the left vent, the cardioplegia needle is reinserted into the aortic root at the end of the intracardiac repair phase, connected to a pump line, and used to complete de-airing prior to releasing the aortic cross-clamp.
The del Nido solution obviously has a blood component, which is not favored by teams wishing to provide bloodless surgery to their patients. Accordingly, more short action extracellular and purely crystalloid solutions such as Calafiore have been employed, with the disadvantage of needing to be repeated every 20 minutes and therefore needing to keep the cardioplegia needle in the ascending aorta during the entire intracardiac repair. Alternatively, with the goal of providing bloodless (transfusion-free) surgery, Bretschneider’s intracellular crystalloid solution provides longer cardioplegia-free intervals, and is currently used for all our mini right axillary repairs.
Finally, in larger patients (>50 k), the aorta may be quite remote and clamping or cardioplegia administration judged hazardous or cumbersome. In these cases, cooling to moderate hypothermia at 32–34 degrees Celsius is used with induced VF for the intracardiac phase of the repair, with either spontaneous defibrillation occurring at rewarming or defibrillation using paddles at the end of repair/intracardiac shunt closure. When available, special fibrillation pads are attached to the external ventricular free wall to induce fibrillation, and secured with a stitch, to avoid inadvertent defibrillation if contact is lost. Alternatively, and extensively used during humanitarian missions to middle income or developing countries, temporary pacing wires may be secured to the ventricular free wall, and overdrive atrial pacing achieved using high frequency (>300 bpm) stimulation to the ventricular electrode and thereby induce VF. Safe repairs without post-operative ventricular dysfunction have been achieved with induced VF times of up to 45 minutes.
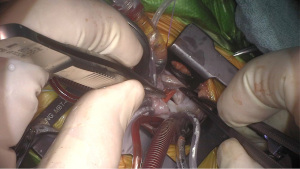
After right atrial access, various repairs are performed as they otherwise would be through a standard median sternotomy, with certain technical tips and tricks added to improve exposure through the small incision. The surgical site including cannulae, the aortic cross-clamp, and exposure showing the tricuspid valve after right atriotomy are shown in Figure 5. Repair of a VSD with a patch after detaching the anterior leaflet of the tricuspid valve is shown in Figure 6. Weaning from bypass, decannulation and hemostasis are all standard and equivalent to a repair from the front. Regarding thoracotomy closure, particular care is given to respecting the intercostal space, which requires attention to technique at the time of chest opening, as mentioned before. Chest closure is performed by gently reapproximating the inferior rib and periosteal cuff of tissue from the intercostal space above, thereby avoiding rib fusion, and contributing to more easy post-operative pain management. All repairs are evaluated by transesophageal echocardiography and deemed satisfactory prior to decannulation and planned extubation before leaving the operating room.

Fast-tracking and pain management
As almost all scheduled defects involve elective surgery in otherwise healthy patients undergoing biventricular repair and no pre-operative respiratory compromise or mechanical ventilation, in-theatre extubation immediately after surgery is an inherent part of the minimal invasive protocol and planned accordingly in concert with anesthesiology and perfusion. Post-operative pain control has evolved over time, and is strongly influenced by institutional anesthesia preference and protocols, using 3 different methods in our experience.
Initially, from 2005–2012, inter (para)costal catheters were placed under direct vision by the surgeon along the inferior border of an already opened intercostal space and tunneled toward the outside, prior to thoracotomy closure. Continuous local anesthesia infusion was given using bupivacaine 0.125% at 0.2 mg/kg/h until after chest tube removal or at a maximum of 48 hours post-operatively, whichever came first.
From 2013–2020, the protocol included the pre-operative placement of a thoracic epidural catheter prior to skin incision under transesophageal sonography (TEE) guidance, low-dose intraoperative opioid use, targeted early extubation, early feeding, scheduled non-opioid analgesic medications in the immediate postoperative period, and early mobilization. The epidural interspace was accessed by the loss of resistance technique in the lower thoracic interspace (T6-L1). A stimulating catheter was then advanced under TEE guidance, and the catheter tip location was advanced to the level of the aortic valve in a short-axis view. The expansion of the epidural space could also be observed with the injection of saline via the epidural catheter. The catheter was then secured, and a bolus of 0.4 mg/kg of 0.2% ropivacaine was given, followed by a continuous infusion (0.2 mg/kg/h) for 48 hours. The protocol addressed concerns regarding systemic heparinization during the procedure and postoperative catheter care. Importantly, at least one hour was allowed between insertion of the epidural catheter and systemic heparinization required for cardiopulmonary bypass. If the epidural was declined or unsuccessful (more than three attempts) or was deemed unsafe (aspiration of blood during localization), the surgeon placed an intrapleural para-costal catheter under direct vision before closing the thoracotomy, as mentioned before in the era 2005–2012.
Finally, since 2020, serratus blocks have been used at the end of the operation, prior to extubation, using the still sterile operative field after thoracotomy closure. One time intra-fascia (intramuscular) ropivacaine (0.375%) is injected using ultrasound guidance (2.5 mL/kg), divided into 2 doses:
- half in the fascia between the anterior border of the rib and the anterior serratus muscle (deep injection) and
- half in the fascia between the latissimus dorsi and serratus anterior muscles (superficial injection).
The serratus block is expected to provide adequate pain relief for 8 hours, and complements other standard post-operative breakthrough analgesia. Using the latter 2 protocols and methods of intra-operative pain management, namely the thoracic epidural catheter and the serratus block, in theatre extubation has been achieved in nearly 90% of our patients (19). While it may be argued that the post-operative pain management protocols are more aggressive involving the use of catheters or local anesthetic injections, they have allowed better pain management as assessed by a subjective parent and patient questionary (4), but more importantly, diminished the use of opioid use.
Post-operative care/discharge without restrictions
After a satisfactory repair and the usual one to 2 days of intensive care stay followed by an average 3–4 day hospital stay (2,4,18-21), no restrictions to physical activity are imposed on the children. There have been no instances of wound dehiscence or deep axillary wound infections. If comfortable and satisfied with their own degree of right arm and shoulder mobility, the patients are encouraged to be as active as desired. Since the approach is through an intercostal space and does not cut through any bone, there is no long healing process of 4–6 weeks depending on the patient’s age, as is after repairs through a median sternotomy, and correspondingly no threat of deep sternal wound infection or mediastinitis. We have found this one of the most appreciated post-operative facts not only for the children/patients themselves, but also for the parents, as they are quite relieved not to need special handling instructions, allow their children to mobilize freely at home, play, ride bicycles, etc., and thereby allowing the quickest potential functional recovery for kids to become kids again.
Conclusions
Open heart repairs for the most common congenital defects are routinely performed through a midline median sternotomy and achieve excellent clinical outcomes. However, lengths of intensive care and hospital stay, age-dependent duration of sternum healing and the corresponding limitation to physical activity until full healing has occurred, the possibility of deep sternal wound infection and associated chronic pain, respiratory dysfunction, and most cosmetically obvious, the visible midline chest wound, are relative caveats of the sternotomy approach. Traditional surgical training provided almost everywhere has allowed the excellent results through midline sternotomy to be reproducible, even in relatively inexperienced surgical hands, developing programs or health systems. However, in the general context of elective surgery, increasing patient demand is being made for less invasive surgical procedures: less invasive with regards to practical aspects (shorter length of stay or even ambulatory surgery), functional maintenance (faster recovery and return to daily activities), and reduced psychological burdens (hidden incision, better cosmetic appearance, losing the stigma of being a heart patient). In the last 15 years, the ever-increasing spread of minimal invasive concepts, and increasing comfort with minimal invasive approaches and instruments derived mostly from the adult cardiac surgery experience, have encouraged more congenital heart teams to apply the concept to pediatric heart repairs (7,9,10,23). While requiring a certain initial learning curve as with all surgical approaches and techniques, the mini right mid-axillary approach, like other right chest incisions, is reproducible, teachable, safe, and yields excellent results (2,4,18-21,23-25).
The authors’ experience and that from other centers (5-7,11-17,22,23) do not reveal true disadvantages per se to the mini thoracotomy approaches, compared to median sternotomy. However, certain words of caution are pertinent, and follow the primary principle applicable to all medicine: “primum non nocere”, first do no harm. As with any “new” approach, results must be at least as good or within reasonable confidence intervals as the established norms already in place, where high standards have been demonstrated for decades, and expectations for near perfect results are appropriately high. In over 320 operations, we recorded a rate of complications and redo surgeries which are comparable to those encountered when repairing the same defects through a median sternotomy: 1 takeback for bleeding, 1 heart block requiring a pacemaker insertion, one phrenic nerve injury, and 3 unplanned early reoperations for residuals. These included a redo scimitar repair, whereby the reimplanted scimitar vein was anastomosed at the hinging point of the interatrial septum and required a septum plasty, and 2 redo patch extensions for inferior sinus venosus ASD repairs. All reoperations were performed through the same right mini axillary thoracotomy and no additional incision was needed. Similar to our experience, the very impressive series by An et al. reported a 0.3% early reoperation rate and no late reoperations in over 1,672 patients (23).
Since current surgical training curriculums available to all congenital heart surgeons allow achieving high standards through a median sternotomy, the additional training and learning curve required for the mini thoracotomy approaches, is not wished by or possible for most. Indeed, since a steep learning curve is not practical or acceptable in today’s atmosphere of great expectations, many understandably ask why an “easy” operation should be turned into something more “difficult”. Through mini right thoracotomies, space is limited, each step must be perfect as the margin for error is small and repeated maneuvers should be reduced to a minimum, and therefore each step takes more time. However, with gained experience, all surgical steps become more routine and expeditious, and the intra-operative comfort zone expands. With regards to the indication of lesions/defects being treated through these approaches, a certain philosophical word of caution is suggested by the authors: if a defect cannot be “cured” with one operation, i.e., the rate of reoperation for recurrent or residual lesions is high and predictable (such as left atrio-ventricular valve regurgitation after complete atrio-ventricular canal repair, even via sternotomy), the right thoracotomy approach is probably not a good option. As redo cardiac operations through the right chest are not yet a routine part of anyone’s surgical curriculum, anticipating a redo repair through the same mini right incision should probably preclude the first thoracotomy to begin with: i.e., if a future redo operation will mean a second median sternotomy incision, starting with the right mini thoracotomy incision is not doing the patient a favor, and not recommended.
Multiple centers worldwide have compared their results repairing the same diagnoses and categories of heart defects performed either through a traditional median sternotomy or right thoracotomy, to conclude that the results of both approaches in their own hands are indeed similar, thereby showing the safety of the mini right thoracotomy approach (13-17). In a meta-analysis of 7 publications comparing an ASD closure through either a right antero-lateral mini-thoracotomy or median sternotomy including 665 patients, Lei et al. (15) found no difference in success or complication rates. Although the mini-thoracotomy group had longer aortic cross-clamp times, their intubation times, length of intensive care and hospital stays, and lengths of incision were shorter, suggesting faster functional recovery and better cosmetic results. Similarly, in a review of 6 case control studies comparing the same two approaches and enrolling 852 patients between 1997 and 2011 with a wider spectrum of repaired congenital heart defects, Ding et al. found slightly longer cross-clamp and cardiopulmonary bypass times, but shorter intubation and post-operative hospital stay times (16).
More experienced teams have gone so far as to suggest enhanced results through mini right thoracotomies, with advantages over the median sternotomy in the categories of post-operative pain, length of hospital stay, wound healing, and return to functionality (4,19,22). In a recent publication, Said et al. (22) reported their experience in 37 small children with a variety of congenital heart repairs, achieving in theater extubation in all patients, with a median in-hospital stay of 3.3±2 days, and excellent cosmetic and functional results at mid-term follow-up. In the most impressive published series to date, An et al. from Beijing reported on 1,672 patients, operated at a median age of 2.3 years, for 13 different types of primary procedures, with spectacularly consistent results: no mortality or conversions to sternotomy, a 0.3% early reoperation rate, a very minor complication rate, no late reoperations, no thoracic deformity or breast asymmetry, and only one case of mild scoliosis (23). This performance sets a new benchmark in what can be achieved through the mini right vertical infra-axillary incision, and is suggested as a good alternative to performing repairs through a median sternotomy (23).
In conclusion, the physician and public interest and demand for minimal invasive surgical approaches are increasing, and have extended into the pediatric cardiac surgery world. For the elective repair of the most common congenital heart defects, namely an ASD, almost all VSD’s, PAPVR, pAVC with mitral cleft, cor triatriatum and scimitar syndrome, the minimal invasive approaches, and specifically in our hands, the mini right axillary thoracotomy, is proving to be safe and reproducible. The quality of repairs is just as high and durable as those historically performed through median sternotomy, with low complication rates or need for reoperation (4,24). The cosmetic and functional results are excellent and superior to the median sternotomy, with faster recovery times for infants, children, adolescents and young adults. As such, for the repair of the most common congenital heart defects, the mini right axillary thoracotomy approach is standard protocol in ours and centers of experience. In time, with enhanced sharing of knowledge and surgical education, and an internationally increasing number of surgeons and centers feeling comfortable, the approach is gaining more widespread acceptance, and has the potential to become the new norm.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Antonio F. Corno and Jorge D. Salazar) for the column “Pediatric Heart” published in Translational Pediatrics. The article has undergone external peer review.
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-282/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-282/coif). The column “Pediatric Heart” was commissioned by the editorial office without any funding or sponsorship. ADK serves as an unpaid editorial board member of Translational Pediatrics from August 2021 to July 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- European Congenital Heart Surgeons Association Congenital Database. Specific Procedures Harvest, 1999-2022. Available online: www.echsacongenitaldb.org
- Dodge-Khatami A, Salazar JS. Right axillary thoracotomy for transatrial repair of congenital heart defects: VSD, partial AV canal with mitral cleft, PAPVR or Warden, cor triatriatum, and ASD. Oper Techniques Thorac Cardiovasc Surg 2016;20:384-401. [Crossref]
- Schreiber C, Bleiziffer S, Kostolny M, et al. Minimally invasive midaxillary muscle sparing thoracotomy for atrial septal defect closure in prepubescent patients. Ann Thorac Surg 2005;80:673-6. [Crossref] [PubMed]
- Dodge-Khatami J, Dodge-Khatami A. Advantages of a mini right axillary thoracotomy for congenital heart defect repair in children. Cardiol Young 2022;32:276-81. [Crossref] [PubMed]
- Li G, Su J, Fan X, et al. Safety and Efficacy of Ventricular Septal Defect Repair Using a Cosmetic Shorter Right Lateral Thoracotomy on Infants Weighing Less than 5 kg. Heart Lung Circ 2015;24:898-904. [Crossref] [PubMed]
- Liu H, Wang Z, Xia J, et al. Evaluation of Different Minimally Invasive Techniques in Surgical Treatment for Ventricular Septal Defect. Heart Lung Circ 2018;27:365-70. [Crossref] [PubMed]
- Lee T, Weiss AJ, Williams EE, et al. The Right Axillary Incision: A Potential New Standard of Care for Selected Congenital Heart Surgery. Semin Thorac Cardiovasc Surg 2018;30:310-6. [Crossref] [PubMed]
- Palma G, Giordano R, Russolillo V, et al. Anterolateral minithoracotomies for the radical correction of congenital heart diseases. Tex Heart Inst J 2009;36:575-9. [PubMed]
- Iribarne A, Easterwood R, Chan EY, et al. The golden age of minimally invasive cardiothoracic surgery: current and future perspectives. Future Cardiol 2011;7:333-46. [Crossref] [PubMed]
- Alsarraj MK, Nellis JR, Vekstein AM, et al. Borrowing from Adult Cardiac Surgeons-Bringing Congenital Heart Surgery Up to Speed in the Minimally Invasive Era. Innovations (Phila) 2020;15:101-5. [Crossref] [PubMed]
- Lin PJ, Chang CH, Chu JJ, et al. Minimally invasive cardiac surgical techniques in the closure of ventricular septal defect: an alternative approach. Ann Thorac Surg 1998;65:165-9; discussion 169-70. [Crossref] [PubMed]
- Baharestani B, Rezaei S, Jalili Shahdashti F, et al. Experiences in surgical closure of atrial septal defect with anterior mini-thoracotomy approach. J Cardiovasc Thorac Res 2014;6:181-4. [Crossref] [PubMed]
- Hong ZN, Chen Q, Lin ZW, et al. Surgical repair via submammary thoracotomy, right axillary thoracotomy and median sternotomy for ventricular septal defects. J Cardiothorac Surg 2018;13:47. [Crossref] [PubMed]
- Hu CX, Tan J, Chen S, et al. Comparison of clinical outcomes and postoperative recovery between two open heart surgeries: minimally invasive right subaxillary vertical thoracomy and traditional median sternotomy. Asian Pac J Trop Med 2014;7:625-9. [Crossref] [PubMed]
- Lei YQ, Liu JF, Xie WP, et al. Anterolateral minithoracotomy versus median sternotomy for the surgical treatment of atrial septal defects: a meta-analysis and systematic review. J Cardiothorac Surg 2021;16:266. [Crossref] [PubMed]
- Ding C, Wang C, Dong A, et al. Anterolateral minithoracotomy versus median sternotomy for the treatment of congenital heart defects: a meta-analysis and systematic review. J Cardiothorac Surg 2012;7:43. [Crossref] [PubMed]
- Chang CH, Lin PJ, Chu JJ, et al. Surgical closure of atrial septal defect. Minimally invasive cardiac surgery or median sternotomy? Surg Endosc 1998;12:820-4. [Crossref] [PubMed]
- Dave HH, Comber M, Solinger T, et al. Mid-term results of right axillary incision for the repair of a wide range of congenital cardiac defects. Eur J Cardiothorac Surg 2009;35:864-9; discussion 869-70. [Crossref] [PubMed]
- Dodge-Khatami J, Noor R, Riggs KW, et al. Mini right axillary thoracotomy for congenital heart defect repair can become a safe surgical routine. Cardiol Young 2022; Epub ahead of print. [Crossref] [PubMed]
- Prêtre R, Kadner A, Dave H, et al. Right axillary incision: a cosmetically superior approach to repair a wide range of congenital cardiac defects. J Thorac Cardiovasc Surg 2005;130:277-81. [Crossref] [PubMed]
- Kadner A, Dodge-Khatami A, Dave H, et al. Closure of restrictive ventricular septal defects through a right axillary thoracotomy. Heart Surg Forum 2006;9:E836-9. [Crossref] [PubMed]
- Said SM, Greathouse KC, McCarthy CM, et al. Safety and Efficacy of Right Axillary Thoracotomy for Repair of Congenital Heart Defects in Children. World J Pediatr Congenit Heart Surg 2023;14:47-54. [Crossref] [PubMed]
- An K, Li S, Yan J, et al. Minimal Right Vertical Infra-axillary Incision for Repair of Congenital Heart Defects. Ann Thorac Surg 2022;113:896-902. [Crossref] [PubMed]
- Yang X, Hu Y, Dong J, et al. Rightvertical axillary incision for atrial septal defect: a propensity score matched study. J Cardiothorac Surg 2022;17:256. [Crossref] [PubMed]
- Dodge-Khatami A, Salazar J. Right Axillary Thoracotomy for Transatrial Repair of a Wide Range of Congenital Heart Defects. April 12, 2016 [cited 2023 Aug 16]. Available online: https://www.ctsnet.org/article/right-axillary-thoracotomy-transatrial-repair-wide-range-congenital-heart-defects
- Dodge-Khatami A. Minimal Invasive Right Axillary Approach for the Repair of Congenital Heart Defects in Children. April 15, 2020 [cited 2023 Aug 16]. Available online: https://www.youtube.com/watch?v=ihnfQGiVDsY&t


