
Identification of a novel splicing variant in the phosphatidylinositol glycan class S (PIGS) gene that is associated with early onset epilepsy, severe developmental delay, and ventricular septal defect: a case report
Highlight box
Key findings
• The c. 935-6C>G variant, which leads to the skipping of exon 10, was identified in the phosphatidylinositol glycan class S (PIGS) gene.
What is known and what is new?
• To date, 13 cases with PIGS variants have been reported. These patients showed severe global developmental delay, seizures, hypotonia, and some dysmorphic features. Eleven PIGS variants have been identified, including only one canonical splicing variant.
• This case study describes a boy with early onset refractory seizures, which was successfully controlled after taking vitamin B6. To the best of our knowledge, this is the first report of ventricular septal defect in the heart of a patient with PIGS variants. The skipping of exon 10 was observed in the proband by RNA analysis and the pathogenicity of the splicing variant c. 935-6C>G was confirmed.
What is the implication and what should change now?
• The mechanisms by which PIGS variants cause intellectual deficiency or epilepsy warrant further clarification.
Introduction
Glycosylphosphatidylinositols (GPIs) are glycolipids that act as membrane anchors of many cell surface proteins. GPI biosynthesis begins on the cytoplasmic side of the endoplasmic reticulum (ER) and involves multiple modifications and attachment to the GPI anchor proteins (GPI-APs) (1). During this period, the GPI transamidase replaces the C-terminal GPI attachment signal peptide of the protein with a preassembled GPI (2,3). Phosphatidylinositol glycan class S (PIGS) encodes an essential component of the multi-subunit, membrane-bound, GPI transamidase, which comprises 4 other proteins including PIGK, GPAA1 (glycosylphosphatidylinositol anchor attachment 1), PIGT and PIGU (4).
Defects in genes that encode portions of the GPI biosynthetic pathway represent a congenital disorders of glycosylation (CDG), termed inherited GPI deficiencies (IGDs). In 2018, six patients with PIGS mutations from three unrelated families were reported to have severe global developmental delay, seizures, hypotonia, weakness, ataxia, and dysmorphic facial features (5). Two years later, another Chinese boy with infantile spasms, as well as hearing and visual impairment, expanded the phenotype of patients with PIGS variants (6). In 2020, six additional cases provided evidence that PIGS variants may be responsible for early onset epileptic developmental encephalopathy (DEE) (7). To date, eleven variants in PIGS have been reported, including only one canonical splicing variant (5-7).
Herein, we identified a novel splicing variant, c.935-6C>G, of PIGS which led to the skipping of exon 10. Furthermore, this investigation is the first to report a ventricular septal defect in patients with PIGS variants and review the clinical and genetic features of PIGS. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-317/rc).
Case presentation
This case report describes a male who was the second child born to non-consanguineous parents. The patient was born at full term by vaginal delivery with a birth weight of 3,850 g. His mother experienced some vaginal bleeding in the first trimester. Amniocentesis was performed during the second trimester for a thickened nuchal fold, and the cytogenetics results were normal. There was no family history of epilepsy nor developmental delay.
At the age of 2 months, the child experienced his first afebrile focal seizure, manifesting in head deviations to the left side with blue lips and no response. Ten days later, he had the same seizure. Although the interictal electroencephalography (EEG) was normal, levetiracetam was administered at five months old, with the dosage gradually increasing to 45 mg/kg/day. The seizures were not well controlled, occurred in cluster up to 10 times per day, and frequently lasted longer than 5 minutes with fevers or infections. At the age of 8 months, he developed myoclonic jerks. His EEG at 8 months revealed an increase in slow waves over the posterior regions associated with multifocal epileptic discharges in the bilateral frontal, central, and parietal regions. Multiple anti-seizure medications, including sodium valproate (40 mg/kg/day) and topiramate (5.6 mg/kg/day) were prescribed at 9 months of age, but the seizures persisted, with one or two episodes every month. After administration of daily oral pyridoxine (7 mg/kg/day), the patient was more active and gradually became seizure-free. At the last visit, he had not experienced a seizure for half a year. Interruption of pyridoxine administration has not been attempted due to certain concerns.
The patient was noted to have developmental delay at 6 months. He could not control his head, but he laughed loudly after months. At 13 months he could hold up his head for a few seconds and rolls from belly to back and vice versa. At the age of 22 months, he could not speak any words but could sit independently. Lack of eye contact was obvious during further follow-up consultations.
On examination at 6 months old, he had mild hypotonia with profound horizontal nystagmus and esotropia. In addition, it was noted that he had mild facial dysmorphism and normal head circumference, including almond-shaped palpebral fissures, arched and long eyebrows, and frontal bossing. He also presented with a ventricular septal defect in the heart and hepatomegaly. On the last examination at the age of 22 months, he had severe hypotonia with less severe nystagmus than previously noted.
Prior laboratory examinations including blood cell count, renal and liver function, total bilirubin, uric acid, albumin, lactate, ammonia, amino acids, and blood gasses were all normal. Urine organic acids were also normal. Brain magnetic resonance imaging (MRI) of the cavum septum pellucidum was unremarkable. Visual and brainstem auditory evoked potentials were performed unsuccessfully due to non-cooperation. Serum alkaline phosphatase levels were normal for his age.
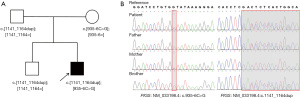
Trio whole exome sequencing (WES) was performed using the NovaSeq 6000 Sequencing platform. Sanger sequencing was performed to validate the variations identified by WES. A sample obtained from the patient’s brother was also analyzed by Sanger sequencing. Compound heterozygous variants (NM_0033198.4 c.935-6C>G and c.1141_1164dup) in the PIGS gene were identified in the proband (Figure 1). The c.1141_1164dup (p. Asp381_Val388dup) was inherited from his father and has been previously described in the Genome Aggregation Database (gnomAD) in a heterozygous carrier state in five individuals of East Asian ethnicity. In addition, his unaffected older brother was heterozygous with the c.1141_1164dup variant. The c.1141_1164dup has been previously reported in two unrelated Chinese patients with PIGS variants (6,7). The c.935-6C>G variant originated from the maternal side and has been reported in the gnomAD in two heterozygous carriers who were also from East Asia.
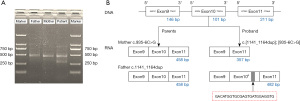
The Topaht software was used for junction analysis, and the number and proportion of new splice junctions in the transcriptome data was obtained by comparing known splice junctions. With this information, different splicing patterns including skipped exons, alternative 5' splice sites, alternative 3' splice sites, mutually exclusive exons, and retained introns in different samples can be suggested using the rMATS software and correlated with their biological characteristics. Computational splice analysis of the c. 935-6C>G variant predicted the following two possible effects that prompted further RNA studies: (I) skipping of exon 10; and (II) mutually exclusive exons between exon 6 and exon 8.
Reverse transcription polymerase chain reaction (RT-PCR) was performed to identify the splicing alternations. Primer sets (forward primer: 5'-GTGCTGCCTCCTTGTACCCTG-3'; reverse primer: 5'-CGTCGTCCTTAATGACAATG-3') were designed to amplify the complementary DNA. The PCR products were analyzed by 2.0% agarose gel electrophoresis and sequenced. In vitro RNA analysis revealed a 482-bp amplicon from exon 9 to exon 11 in the patient, as well as a 357-bp amplicon only in exon 9 and exon 11 (Figure 2). The amplicons in the proband’s parents revealed in exon 9, 10, and 11 (458-bp) (Figure 2). This likely indicated alternative splicing.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This current study expanded upon the phenotypic and genotypic spectrum of PIGS variants. Using RNA analysis, we clarified that the novel variant (c. 935-6C>G) leads to exon skipping. To the best of our knowledge, this is the first report of ventricular septal defect in the heart of patients with PIGS variants.
The epilepsy due to PIGS variants is almost early infantile-onset and refractory. To date, 14 cases with PIGS variants have been reported (Table 1), but two cases were terminated during pregnancy. Of the 12 patients with epilepsy, 11 patients had detailed data related to age at seizure onset (range 1 to 12 months). Onset before 3 months of age occurred in 8 patients. In 10 of 12 patients, information regarding the initial seizure type was available. The most common presentation was focal seizure in 7 cases, febrile seizure in 2 cases, and epileptic spasm in one case. Additional seizure types including myoclonic seizures, tonic-clonic seizures, tonic spasms, and status epilepticus were recorded. The epilepsy in 10 cases was refractory, and 2 cases achieved partial control. The most common antiseizure medication was levetiracetam, which was administered in 9 cases, while valproate or topiramate were administered in 5 cases.
Table 1
| Features | This study | Nguyen et al. (5) | Efthymiou et al. (7) | Zhang et al. (6) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | |||||
| Family | 1 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 1 | ||||||||
| Gender | M | M | M | M | M | F | F | M | M | M | M | F | F | M | ||||
| Ethnicity | Chinese | European | Mexican | European | Pakistan | Pakistan | Pakistan | Egyptian | Chinese | Chinese | ||||||||
| Premature death/age at death | N | N | N | N | N | Terminated pregnancy | Terminated pregnancy | N | Y/1.9 y | Y/2 y | − | Y/1 y | − | Y/15 mo | ||||
| Epilepsy | Y | Y | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | ||||
| Age at seizure onset | 2 mo | 8 mo | 1 y | NA | 8 mo | NA | NA | 1 mo | 1 mo | 2 mo | 2 mo | 1 mo | 1 mo | 2 mo | ||||
| First Seizure types | Focal | FS | FS | NA | NA | NA | NA | Multifocal clonic, myoclonic | Multifocal clonic | Focal/multifocal clonic | Focal | Focal | Multifocal | Infantile spasms | ||||
| Seizure evolution | Myoclonic | LGS | Afebrile | NA | NA | NA | NA | Myoclonic | Tonic-clonic | Tonic spasms, Tonic-clonic | Tonic-clonic | Tonic-clonic, myoclonic | Multifocal | NA | ||||
| Seizure frequency | Up to 10/day | NA | NA | NA | NA | NA | NA | 3–4/day reduced to 1-2/mo | Multiple/day | 3-4/day increased to multiple/day | 1/mo | 1/day | 10/day | Up to 10/day | ||||
| Status epilepticus | Yes, fevers or infections | NA | NA | NA | NA | NA | NA | N | Y | Y | Y, 4 times per year | Y, several episodes per month | Y, multiple episodes | Y | ||||
| Medications employed | VPA, LEV, TPM | LEV, CLB, Rufinamide | NA | NA | VPA | NA | NA | LEV, NZP, TPM | LEV, TPM, CLZ | LEV, CBZ, VPA | VPA, LEV, TPM, CLZ | VPA, LEV, TPM, PHT | CLB, LEV, PER, DZP, ZNS, CAN | VPA, LEV, ACTH | ||||
| Response to ASM | Refractory | Partial | Partial | Refractory | Refractory | NA | NA | Refractory | Refractory | Refractory | Refractory | Refractory | Refractory | Refractory | ||||
| B6 trial and results | Y, seizure-free | N | N | Y, seizure-free | Yes, NA | NA | NA | Y, decreased frequency | N | N | N | N | Y, improved development | N | ||||
| DD/ID | Y | Y | Y | Y | Y | NA | NA | Y | Y | Y | Y | Y | Y | Y | ||||
| Hypotonia | Y | Y | Y | Y | Y | NA | NA | N | N | N | Y | Y | Y | Y | ||||
| Other neurologic features | N | Ataxia | Ataxia | Ataxia, hyporeflexia | Ataxia, hyporeflexia | NA | NA | N | N | Appendicular spasticity | Hyperreflexia | Spasticity | N | Hyporeflexia | ||||
| Congenital anomalies | Ventricular septal defect | N | N | Umbilical and inguinal hernias, Cryptorchidism, Microcephaly | Microcephaly | N | Renal glomerular cysts | Microcephaly | Microcephaly | Microcephaly | Microcephaly | Microcephaly | Asymmetric kidneys, Microcephaly | N | ||||
| Hearing loss | NA | Y | Y | N | NA | NA | NA | Y | N | N | N | N | N | Y | ||||
| Impaired vision | NA | Y, cortical blindness | Y, cortical blindness | Y | N | NA | NA | Y | Y | N | N | N | Y, cortical blindness | Y, cortical blindness | ||||
| Other ophthalmologic findings | Nystagmus, strabismus | Nystagmus | Nystagmus | Nystagmus | N | N | N | N | N | N | N | N | Astigmatism | N | ||||
| Variants (NM_00331984) | c.1141_1164dup | c.108G>A | c.108G>A | c.1316_1352delinsGGTTGCT | c.1316_1352delinsGGTTGCT | c.923A>G | c.923A>G | c.174G>C | c.174G>C | c.1070G>A | c.986C>G | c.986C>G | c.1141_1164dup | c.1141_1164dup | ||||
| c.935-6C>G | c.101T>C | c.101T>C | c.468+1G>C | c.468+1G>C | c.734G>A | c.148C>T | ||||||||||||
| Inheritance/Consanguinity | Com het/N | Com het/ N | Com het/N | Hom/NA | Hom/NA | Com het/N | Com het/N | Hom/Y | Hom/Y | Hom/Y | Hom/Y | Hom/Y | Com het/ N | Com het/N | ||||
PIGS, phosphatidylinositol glycan class S;F, female; M, male; mo, months; NA, not available; y, years; Y, Yes; N, No; FS, Febrile seizures; LGS, Lennox-Gastaut syndrome; VPA, Valproate; LEV, Levetiracetam; TPM, Topiramate; CLB, Clobazam; NZP, Nitrazepam; CLZ, Clonazepam; CBZ, Carbamazepine; PHT, Phenytoin; PER, Perampanel; DZP, Diazepam; ZNS, Zonisamide; CAN, Cannabidiol; ACTH, adrenocorticotropic hormone; ASM, anti seizure medications; DD, Developmental delay; ID, Intellectual disability; Com het, compound heterozygous; Hom, homozygous.
Our case subject experienced very severe seizures and hypotonia. Although the types and frequencies of the seizures were progressive, his EEG initially showed no seizure activity. This was similar with other cases of PIGT variations (8-10) and it may be a differentiating characteristic with other early infantile epileptic encephalopathy (EIEE). The patient had mild hypotonia in the early stage, which gradually developed to severe hypotonia during follow-up.
Multiple malformations, including brachydactyly, clinodactyly, pectus carinatum, and glomerular cysts, have been reported in the cases with PIGS variants [5, 7]. However, this case report is the first to report ventricular septal defect in heart of a patient with PIGS variant.
Epilepsy due to inherited GPI defected might be vitamin B6 responsive. However, it remains unclear whether PIGS associated epilepsy can be considered as a pyridoxine-responsive epilepsy. There have been 5 reported PIGS cases who received vitamin B6 therapy. Pyridoxine alleviated seizures in two of the affected probands [5, 7] and improved development in one patient with a PIGS variant (7). The patient in our case study may benefit from vitamin B6 administration.
This study expanded the genotypes of PIGS and identified a splicing variant using RNA analysis. Eleven variants, including 5 missense variants, 3 nonsense variants, 1 in-frame variant with a deletion and insertion, 1 in-frame variant with a duplication, and 1 canonical splicing variant have been reported in PIGS cases (5-7). Indeed, there may appear to be some hotspots for PIGS variants in Eastern Asian. Analysis of the gnomAD revealed that both the c.1141_1164dup and the c.935-6C>G variants were seen in heterozygous carriers with East Asian origins, and the former variant has also been reported in two unrelated patients with Chinese origin (6,7).
The phenotypes of PIGS are characterized as DEE with early-infantile-onset epilepsy. The mechanisms by which PIGS variants and GPI-APs are linked to intellectual deficiency or epilepsy warrant further clarification.
Conclusions
The c. 935-6C>G variant, which leads to the skipping of exon 10, enlarges the variant spectrum in the PIGS gene. Furthermore, the phenotypic spectrum of PIGS variants is also expanded, with ventricular septal defect in the heart being reported for the first time in patients with PIGS variants.
Acknowledgments
The authors are grateful to the family who participated in the study. The authors would like to thank Amplicongene (Shanghai, China) for the helpful bioinformatics analysis and Xue Yang (Children’s Hospital of Fudan University, Shanghai, China) for her assistance with the collection of specimens.
Funding: The study was funded by Key Development Program of Children’s Hospital of Fudan University (No. EK2022ZX01).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-317/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-317/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-317/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Almeida A, Layton M, Karadimitris A. Inherited glycosylphosphatidyl inositol deficiency: a treatable CDG. Biochim Biophys Acta 2009;1792:874-80. [Crossref] [PubMed]
- Ohishi K, Inoue N, Kinoshita T. PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. EMBO J 2001;20:4088-98. [Crossref] [PubMed]
- Ohishi K, Nagamune K, Maeda Y, et al. Two subunits of glycosylphosphatidylinositol transamidase, GPI8 and PIG-T, form a functionally important intermolecular disulfide bridge. J Biol Chem 2003;278:13959-67. [Crossref] [PubMed]
- Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J Biochem 2008;144:287-94. [Crossref] [PubMed]
- Nguyen TTM, Murakami Y, Wigby KM, et al. Mutations in PIGS, Encoding a GPI Transamidase, Cause a Neurological Syndrome Ranging from Fetal Akinesia to Epileptic Encephalopathy. Am J Hum Genet 2018;103:602-11. [Crossref] [PubMed]
- Zhang L, Mao X, Long H, et al. Compound Heterozygous PIGS Variants Associated With Infantile Spasm, Global Developmental Delay, Hearing Loss, Visual Impairment, and Hypotonia. Front Genet 2020;11:564. [Crossref] [PubMed]
- Efthymiou S, Dutra-Clarke M, Maroofian R, et al. Expanding the phenotype of PIGS-associated early onset epileptic developmental encephalopathy. Epilepsia 2021;62:e35-41. [Crossref] [PubMed]
- Kvarnung M, Nilsson D, Lindstrand A, et al. A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J Med Genet 2013;50:521-8. [Crossref] [PubMed]
- Lam C, Golas GA, Davids M, et al. Expanding the clinical and molecular characteristics of PIGT-CDG, a disorder of glycosylphosphatidylinositol anchors. Mol Genet Metab 2015;115:128-40. [Crossref] [PubMed]
- Nakashima M, Kashii H, Murakami Y, et al. Novel compound heterozygous PIGT mutations caused multiple congenital anomalies-hypotonia-seizures syndrome 3. Neurogenetics 2014;15:193-200. [Crossref] [PubMed]


