
Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis
Introduction
Hand, foot, and mouth disease (HFMD) caused by coxsackievirus A6 (CV-A6) infection is widely known as atypical HFMD. Compared with traditional HFMD, it is more likely to present as bullous lesions and diverse rashes, and it has a high rate of misdiagnosis (1). Since it first broke out in Finland in 2008, it has become prevalent in many parts of the world (2), imposing a significant public health burden. According to clinical studies, since 2013, CV-A6 has been the one of the main pathogens responsible for HFMD in many Chinese provinces and cities (3-5). Although some studies on CV-A6 have been reported, most of them focus on epidemiology. There is little complete analysis of the clinical characteristics including the morphology and distribution of rashes of atypical HFMD caused by CV-A6, and there is no grouping analysis according to age. We thus sought to conduct a retrospective analysis of the clinical data of 68 children with bullous lesions caused by CV-A6 who were hospitalized in the Children’s Hospital of Soochow University during the period from 2018 to 2020. Findings on the clinical characteristics including the morphologies and distribution of rashes, the details of fever, the presence or absence of onychomadesis, and laboratory test results according to the age group categories of infant (<1 year), toddler (1–<3 years), and preschool (3–<6 years) are presented and discussed, with a view to providing a basis for the diagnosis, treatment, prevention, and control of the disease. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-352/rc).
Methods
Participants
A total of 68 children who were hospitalized in the Infectious Diseases Department of the Children’s Hospital of Soochow University from January 2018 to December 2020 were included in this study. The children’s throat swab samples were all positive for CV-A6 virus nucleic acid according to reverse transcription-polymerase chain reaction. All diagnoses of HFMD conformed to the 2018 Chinese guidelines for the diagnosis and treatment of HFMD (6). The following inclusion criteria, which are relevant to the diagnosis of atypical HFMD according to the related literature (4,7-9), were also applied: large herpes (diameter >0.5 cm) and rashes distributed mostly in places other than the hands, feet, and buttocks. Two specialists were required simultaneously to make a clinical diagnosis. All children were treated with antiviral and supportive symptomatic treatment and had a good prognosis. The exclusion criteria were as follows: (I) a special drug history or skin allergy that may cause skin lesions; and (II) the presence of other diseases, such as immune deficiency or a tumor, that may affect the clinical manifestation of HFMD. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the ethics committee of the Children’s Hospital of Soochow University (No. 2020zs027), and the informed consent was obtained from the patients’ parents.
Research methods
This study used a retrospective design of case series. Data collected for analysis were as follows: (I) epidemiological data, including age, sex, month of HFMD onset, and contact history; (II) clinical manifestations of HFMD, including shape and location of rashes, pain and itching of skin lesions, peak fever, fever course, respiratory manifestations such as cough and runny nose, gastrointestinal manifestations such as vomiting and diarrhea, nervous system involvement (if relevant), days of hospitalization, and the presence or absence of onychomadesis during follow-up; and (III) laboratory examination, including white blood cell (WBC) count, C-reactive protein (CRP), liver function, and serum creatine kinase MB isoenzyme (CK-MB).
Statistical analysis
Data were processed using SPSS v. 23.0 statistical software (IBM Corp., Armonk, NY, USA). Measurement data that conformed to a normal distribution and had a homogeneity of variance are expressed as , and analysis of variance was used for comparison between groups. Count data were compared using the χ2 test or Fisher’s test. The threshold of significance was P<0.05.
Results
Epidemiological analysis
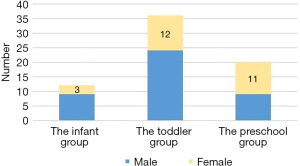
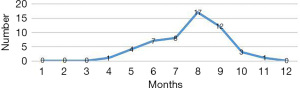
The 68 children enrolled in the study included 42 males and 26 females, with a male to female ratio of 1.62:1. The children ranged in age from 4 months to 5 years and 2 months, with the average age being 1.98±1.03 years. The children were divided into 3 groups according to age: the infant group (<1 year; n=12), the toddler group (1–<3 years; n=36), and the preschool group (3–<6 years; n=20). The sex composition of each group is shown in Figure 1. There were more males than females in the infant and toddler groups, while the opposite was observed in the preschool group. The peak months of disease onset were from June to September, and there were no cases in the period from January to March or in December. The case numbers for each month are shown in Figure 2. Only 15 (22.1%) cases had a definite contact history with patients with HFMD.
Clinical manifestations
Rash morphology
Among the 68 children with HFMD caused by CV-A6 in this study, various forms of rashes were observed, including macular rash, maculopapular rash, herpes, and vesicles, with 33 cases (32.4%) having more than 3 kinds of rashes simultaneously. Rashes were widely distributed in the mouth, hands, feet, hips, trunk, elbows and knees, auricles, and perioral areas, with 53 cases (77.9%) having rashes in more than 5 locations. The rashes were accompanied by pain and pruritus in 30.8% and 15.4% of cases, respectively. The infant group was more prone to macular and more diverse rash types than the other two groups, with 75.0% of infant patients having more than 3 kinds of rashes; this difference was statistically significant (P<0.001). Both the proportion of patients with rashes in the trunk and the proportion of patients with rashes in more than 5 locations simultaneously were higher in the preschool group than in the other two groups, and these differences were statistically significant (P<0.05). The specific results are shown in Table 1.
Table 1
| Group | N | Rash morphology | Rash site | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macula | Maculopapular | Herpes | Vesicles | ≥3 | Oral mucosa | Hands | Feet | Buttocks | Trunk | Elbows + knees | Peri-mouth | Auricle | ≥5 | |||
| Infant | 12 | 9* | 11 | 12 | 1 | 9* | 11 | 11 | 8 | 10 | 2 | 9 | 11 | 1 | 7 | |
| Toddler | 36 | 11 | 29 | 36 | 5 | 10 | 29 | 36 | 26 | 28 | 1 | 24 | 29 | 9 | 27 | |
| Preschool | 20 | 5 | 20 | 20 | 1 | 6 | 15 | 18 | 17 | 19 | 10* | 15 | 15 | 3 | 19* | |
| χ2 | – | 636.866 | 4.613 | – | 0.987 | 8.612 | 1.224 | 3.992 | 1.717 | 2.771 | 17.567 | 0.537 | 1.224 | 1.599 | 6.392 | |
| P | – | <0.01 | 0.074 | – | 0.761 | 0.015 | 0.592 | 0.099 | 0.451 | 0.297 | <0.01 | 0.769 | 0.590 | 0.540 | 0.031 | |
Data are presented as n. *, a statistically significant difference compared with the other two groups (P<0.05). HFMD, hand, foot, and mouth disease; CV-A6, coxsackievirus A6.
Fever and other symptoms
All 68 children in our study had a fever. There were 13 cases (19.1%) with a peak fever of 38.1–39 ℃, 47 cases (69.1%) with a peak fever of 39.1–40 ℃, and 8 cases (11.8%) with a peak fever of more than 40 ℃. The fever lasted for 2–3 days in 49 cases (72.0%), and only 8 cases (11.8%) had a fever for more than 3 days. The peak fever in the toddler group was 39.24±0.56 ℃, which was lower than that in the other two groups (P=0.033). There was no significant difference in the number of fever days between the three groups. The specific figures are shown in Table 2.
Table 2
| Group | N | Fever peak (℃) | Fever duration (days) | Respiratory symptoms | Hospitalization (days) | Onychomadesis |
|---|---|---|---|---|---|---|
| Infant | 12 | 39.61±0.33 | 2.42±0.79 | 3 | 6.08±2.81 | 6/9 |
| Toddler | 36 | 39.24±0.56* | 2.28±0.91 | 21 | 4.83±1.68* | 25/32 |
| Preschool | 20 | 39.56±0.53 | 2.60±1.05 | 7 | 5.95±1.32 | 11/17 |
| F/χ2 | – | 3.606 | 0.765 | 5.312 | 3.444 | 1.368 |
| P | – | 0.033 | 0.469 | 0.077 | 0.038 | 0.565 |
Data are presented as n or mean ± standard deviation. *, a statistically significant difference compared with the other two groups (P<0.05). HFMD, hand, foot, and mouth disease; CV-A6, coxsackievirus A6.
In the acute phase, 31 cases (45.6%) had a runny nose and/or mild cough. No cases of pneumonia were reported. There were no significant differences in the proportion of cases with respiratory symptoms between the groups (P>0.05). Eight cases (11.8%) were complicated by gastrointestinal symptoms, such as vomiting and diarrhea. There were 2 cases of headache and 3 cases of convulsion, but none of the children had nervous system involvement. Encephalitis was excluded after cerebrospinal fluid examination.
All the patients were given antiviral treatment and supportive symptomatic treatment after admission. They were discharged upon meeting the following standard: their temperature was stable for more than 2 days and most of their rashes were dry. The average length of hospitalization in the toddler group was 4.83±1.68 days, which was significantly shorter than that in the infant group and the preschool group (P=0.038).
During the follow-up at 2–3 weeks after discharge, 7 cases were lost from the study. Of the remaining 61 cases, 42 cases (61.8%) developed onychomadesis. All the patients with onychomadesis denied having had other disease or nail trauma during the 8 weeks prior to the onset of the nail abnormalities. These 42 children each had 3–8 nails involved: 36 children had onychomadesis only in their fingers, 3 had it in their fingers and toes simultaneously, and 3 had it only in their toes. At the second follow-up 2 weeks later, all 42 children had new nails with smooth surfaces.
Laboratory examinations
Of the 68 children in this study, 34 cases (50.0%) had leukocytosis; all of these cases were in the range of 10–20 ×109/L, and 18 mainly had an increase in neutrophils. No significant difference was observed in the WBC value or the proportion of children with an increased WBC count between the groups (P>0.05). In all, 42 (61.8%) cases had elevated CRP (>8 mg/L). No significant difference was observed in the CRP value or the proportion of children with elevated CRP between the groups (P>0.05). The specific figures are shown in Table 3. Additionally, 27 cases (39.7%) with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) higher than the normal value of 40 U/L were listed as having abnormal liver function. Among them, 1 case had elevated ALT, 25 cases had elevated AST, and 1 case had simultaneous elevation of ALT and AST. One patient had an AST level of 82.9 U/L, while the other abnormal values were within twice the normal range. A comparison between the age groups showed that the ALT value in the infant group was significantly higher than that in the other two groups (P=0.025). The proportion of children with abnormal liver function was 83.3% and 41.7% in the infant and toddler groups, respectively, while that in the preschool group was only 10.0%; these differences were significant (P<0.001). Of the 68 children in the study, 42 (61.8%) had myocardial damage (CK-MB >3.61 ng/mL). Both the CK-MB value and the proportion of children with myocardial damage were significantly higher in the toddler group than in the other two groups (P<0.05). The specific figures are shown in Table 3.
Table 3
| Group | N | WBC | CRP | Liver function | CK-MB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (×109/L) | Increased | Number (mg/L) |
Increased | ALT (U/L) | AST (U/L) | Increased | Number (ng/mL) |
Increased | |||||
| Infant | 12 | 9.64±3.33 | 5 | 8.16±8.75 | 6 | 27.22±14.50a | 8.16±8.75 | 10c | 3.93±1.13 | 5 | |||
| Toddler | 36 | 11.57±4.38 | 21 | 23.05±29.51 | 24 | 20.73±11.06 | 23.05±29.51 | 15c | 4.53±1.03 | 29b | |||
| Preschool | 20 | 10.77±3.75 | 8 | 16.78±25.09 | 12 | 16.40±6.26 | 16.78±21.49 | 2c | 3.45±1.28 | 8 | |||
| F/χ2 | – | 1.797 | 2.133 | 1.690 | 1.096 | 3.904 | 1.690 | 16.970 | 6.075 | 11.447 | |||
| P | – | 0.174 | 0.344 | 0.192 | 0.578 | 0.025 | 0.192 | <0.001 | 0.004 | 0.003 | |||
a, the difference between groups 1 and 3 was statistically significant (P<0.05); b, compared with groups 1 and 3, the difference was statistically significant (P<0.05); c, the differences between the three groups were statistically significant (P<0.05). Group 1: infant group; group 2: toddler group; group 3: preschool group. Data are presented as n or mean ± standard deviation. HFMD, hand, foot, and mouth disease; CV-A6, coxsackievirus A6; WBC, white blood cell; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK-MB, creatine kinase MB isoenzyme.
Discussion
HFMD is an infectious disease caused by an enterovirus. It usually occurs in children under 5 years old and mainly causes fever and rash. In rare instances, it involves the central nervous system, and in severe cases, it can cause death (10). Since 2008, HFMD has been prevalent in many provinces and cities in China. In the first few years, the dominant causes of HFMD were CV-A16 and enterovirus 71 (EV71). Of these 2 viruses, EV71 is the main pathogen responsible for severe cases of HFMD (11). Since the EV71 vaccine was first listed in China in 2016, data monitoring in many Chinese cities has shown that the pathogenic composition of HFMD has changed immensely, and there has been a sharp rise in the detection rate of CV-A6 (12). According to research data of the Beijing Center for Disease Control and Prevention, the proportion of HFMD cases caused by the CV-A6 pathogen in Beijing increased from 15.11% in 2016 to 81.08% in 2020 (13). There have also been reports of CV-A6 outbreaks in Vietnam, Spain, France, the United States, and other countries, and in all of these outbreaks, it was believed that the rashes caused by CV-A6 were more extensive and serious than those caused by the other viruses (2,8,14).
Similar to the other members of the Picornaviridae family, CV-A6 has a single-stranded, positive-sense RNA genome. It can be segregated into 4 genotypes, designated as A, B, C, and D, and further subdivided into the B1 and B2, C1 and C2, and D1, D2, and D3 subgenotypes (15). In recent years, the D3 and E2 strains have become prevalent in China, France, Spain, Japan, and other countries to varying degrees (16,17). Aside from children, CV-A6 can also cause HFMD in adults with normal immune function; therefore, its pathogenicity and disease burden may be underestimated (18).
The present study collected the clinical data of 68 children who were hospitalized with atypical HFMD caused by CV-A6 from 2018 to 2020. Children under the age of 3 accounted for 70.6% of cases, and there were no children over the age of 6. The average age was 1.98±1.03 years old, and the male to female ratio was 1.62:1. Therefore, children with atypical HFMD caused by CV-A6 tended to be younger and were more likely to be male, which is consistent with results reported in other studies (19,20). Overall, the highest incidence of HFMD in Suzhou occurs during the months from May to July (21), while the peak epidemic season of atypical HFMD caused by CV-A6 occurs from June to September, which is relatively delayed. Huang et al. (22) reported that the CV-A6 epidemic season in Taiwan is from July to October. An HFMD monitoring study conducted from 2006 to 2017 in the United Kingdom suggested that the peak months are from July to August for EV71 and from October to December for CV-A6 (23). Such delays in the epidemic season may be related to climate, virus strains, and host factors.
Because the morphology and distribution of rashes are usually taken as the main basis for the clinical diagnosis of HFMD, atypical HFMD introduces some difficulties regarding clinical diagnosis and treatment. In the 68 cases in this study, the rash sites were widely distributed in the mouth, hands, feet, buttocks, trunk, elbows and knees, auricles, and perioral area. Of all the included cases, 53 cases (77.9%) had rashes at 5 or more sites simultaneously, and 25 cases (36.8%) had 3 or more types of rash simultaneously. Therefore, when making a diagnosis of HFMD, in addition to the presence of large herpes, the distribution of rashes should be taken into account, which is essentially consistent with other reports (24,25). In a comparison of age groups, the infant group in our study was more prone to macular rashes and had a more diverse rash morphology than did the other age groups. The proportion of children with rashes distributed in more areas of the body was higher in the preschool group than in the other two age groups. These differences were statistically significant (P<0.05). In this study, rashes were accompanied by pain and itching in 30.8% and 15.4% of cases, respectively. Ji et al. (26) reported that rashes with pain and itching were observed in 20–40% of cases of atypical HFMD. Considering that children in the infant group might not have been able to express pain clearly, data pertaining to pain and pruritus were not compared between the groups.
In this study, fever was reported in all 68 children, with 80.9% of them having a high fever (temperature >39 ℃) and 72% having regression of their fever within 3 days. Li et al. (27) compared the clinical characteristics of children with HFMD caused by CV-A6 and EV71, and found that the peak fever was higher but the fever duration shorter in children with CV-A6 compared to children with EV71. Our study found that the peak fever was markedly lower in the toddler group than in the other groups (P=0.033). Considering their poor resistance, infants may have a higher peak fever than toddlers, while preschool children may have a stronger immune response. However, further expansion of the sample size is needed to verify our results. In this study, there were 2 cases of headache and 3 cases of convulsion. Encephalitis was excluded after cerebrospinal fluid examination, and no serious cases were identified. This is consistent with the observations of Huang et al. (22) in Taiwan and Renert-Yuval et al. (25) in Israel. However, in a study of 2,350 severe HFMD cases in the Guangxi region of China, Ju et al. (24) detected 298 positive cases of CV-A6, and a previous report had also reported severe cases caused by CV-A6 in some other countries (28). Amino acid changes of V174I and T283A at the 2 main positions of the VP1 structural protein of CV-A6 have been reported to potentially be related to the severity of infection (16). As there were no severe cases in our study, we might conclude that not all atypical cases of rash and more virulent virus strains were included in our study. Therefore, the absence of severe cases in this study may be related to the inclusion solely of cases with atypical rash and infection associated with the less virulent virus strains that are prevalent in our region.
The children in this study were followed up 2–3 weeks after discharge. Among the 61 children who were followed up successfully, 42 (68.9%) had onychomadesis, involving 3–8 nails, mostly on their fingers; after 2 weeks, all of the children had new nails with smooth surfaces. Many clinical studies have reported onychomadesis developing in the later stage of HFMD, especially in patients with CV-A6 infection, with such cases usually occurring within 1–2 months after disease onset. For most children, not all the nails are involved (27,29,30). At present, the causes of onychomadesis caused by HFMD are not clear. Reports speculate that certain novel viruses, mostly CV-A6, are the major cause of onychomadesis, and their virulence may damage the nail matrix (27,29). In contrast, direct injury caused by cutaneous lesions of HFMD around the nail matrix is considered to be a minor cause (29).
Of the 68 children in this study, 50.0% had a slight increase in leukocytes and 61.8% had an increase in CRP. There was no evidence of bacterial infection in clinical practice, and all the patients improved without antibiotic treatment. Changes in WBCs and CRP may be related to stress, tissue destruction, and the immune response (31). In our study, 39.7% of the children had abnormal liver enzymes, which in most cases manifest as slightly increased levels of AST. The proportion of children with abnormal liver function in the infant group was 83.3%, that in the toddler group was 41.7%, and that in the preschool group was only 10.0%. The difference between the groups was statistically significant (P<0.001). Also, 61.8% of the children had increased serum CK-MB. Both the levels of CK-MB and the proportion of children with increased CK-MB were significantly higher in the toddler group than in the other two groups (P<0.05). These results indicate that mild organ damage caused by CV-A6 is common. The development of organ function in the preschool children in our study was relatively uneventful; only 10% of them had abnormal liver enzymes, and 40% had myocardial damage.
This study retrospectively analyzed the clinical data of 68 children who were hospitalized from 2018 to 2020 with atypical HFMD caused by CV-A6. The results showed that the disease often occurred in children under 5 years old, especially boys. The peak of the endemic season was from June to September. We found that the distribution of rashes was wider in atypical HFMD than in traditional HFMD, with affected areas including the mouth, hands, feet, hips, trunk, elbow and knee, auricle, and perioral area. In 77.9% of the children, the rashes involved more than 5 locations, especially in the preschool group. Some children’s rashes were accompanied by pain and itching. All 68 children had fever, most had a high fever, and the fever subsided in 2–3 days. Of the children, 45.6% had mild respiratory symptoms, and no critical cases were observed. Among the 61 children who were followed up successfully, 68.9% developed onychomadesis within 2–3 weeks, which most commonly involved the fingers. More than half of the children showed increases in the WBC count and CRP level, but it is doubtful that this was associated with bacterial infection. Mild organ damage was common, especially in the infant group and toddler group.
This study has significance for guiding the diagnosis, treatment, prevention, and control of atypical HFMD caused by CV-A6. However, it was a single-center study and included in its analysis only 68 cases from a 2-year period. The small sample size and the lack of research on relevant mechanisms potentially contributed to the study’s shortcomings.
Acknowledgments
Funding: This study was funded by Suzhou Key Laboratory for Accurate Diagnosis and Treatment of Children’s Infectious Diseases (No. SZS2020310).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-352/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-352/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-352/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the ethics committee of the Children’s Hospital of Soochow University (No. 2020zs027), and the informed consent was obtained from the patients’ parents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kuntz T, Koushk-Jalali B, Tigges C, et al. Atypical variant of hand-foot-mouth disease. Hautarzt. 2019;70:964-68. [Crossref] [PubMed]
- Kimmis BD, Downing C, Tyring S. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis 2018;102:353-6. [PubMed]
- Wang Z, Liu T, Li J, et al. Risk Factors for Hand, Foot, and Mouth Disease Caused by Coxsackievirus A6 in Children under 6 Years of Age in Tianjin, China: a Case-Control Study. Jpn J Infect Dis 2021;74:437-42. [Crossref] [PubMed]
- Jiang H, Zhang Z, Rao Q, et al. The epidemiological characteristics of enterovirus infection before and after the use of enterovirus 71 inactivated vaccine in Kunming, China. Emerg Microbes Infect 2021;10:619-28. [Crossref] [PubMed]
- Wang J, Zhou J, Xie G, et al. The Epidemiological and Clinical Characteristics of Hand, Foot, and Mouth Disease in Hangzhou, China, 2016 to 2018. Clin Pediatr (Phila) 2020;59:656-62. [Crossref] [PubMed]
- Li XW, Ni X, Qian SY, et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J Pediatr 2018;14:437-47. [Crossref] [PubMed]
- Yan X, Zhang ZZ, Yang ZH, et al. Clinical and Etiological Characteristics of Atypical Hand-Foot-and-Mouth Disease in Children from Chongqing, China: A Retrospective Study. Biomed Res Int 2015;2015:802046. [Crossref] [PubMed]
- Justino MCA. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol 2020;126:104307. [Crossref] [PubMed]
- Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr 2015;27:486-91. [Crossref] [PubMed]
- Zhang W, Huang Z, Huang M, et al. Predicting Severe Enterovirus 71-Infected Hand, Foot, and Mouth Disease: Cytokines and Chemokines. Mediators Inflamm 2020;2020:9273241. [Crossref] [PubMed]
- Solomon T, Lewthwaite P, Perera D, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010;10:778-90. [Crossref] [PubMed]
- Yu F, Zhu R, Jia L, et al. Sub-genotype change and recombination of coxsackievirus A6s may be the cause of it being the predominant pathogen for HFMD in children in Beijing, as revealed by analysis of complete genome sequences. Int J Infect Dis 2020;99:156-62. [Crossref] [PubMed]
- Dong SB, Wang XL, Huo D, et al. Epidemiological characteristics of hand, foot and mouth disease among people aged 6 and over in Beijing, 2011-2020. Zhonghua Liu Xing Bing Xue Za Zhi 2022;43:207-12. [PubMed]
- Hoa-Tran TN, Dao ATH, Nguyen AT, et al. Coxsackieviruses A6 and A16 associated with hand, foot, and mouth disease in Vietnam, 2008-2017: Essential information for rational vaccine design. Vaccine 2020;38:8273-85. [Crossref] [PubMed]
- Liu H, Zhang M, Feng C, et al. Characterization of Coxsackievirus A6 Strains Isolated From Children With Hand, Foot, and Mouth Disease. Front Cell Infect Microbiol 2021;11:700191. [Crossref] [PubMed]
- Yang X, Li Y, Zhang C, et al. Clinical features and phylogenetic analysis of severe hand-foot-and-mouth disease caused by Coxsackievirus A6. Infect Genet Evol 2020;77:104054. [Crossref] [PubMed]
- Song Y, Zhang Y, Ji T, et al. Persistent circulation of Coxsackievirus A6 of genotype D3 in mainland of China between 2008 and 2015. Sci Rep 2017;7:5491. [Crossref] [PubMed]
- Cordeiro E. Exuberant Hand-Foot-Mouth Disease: An Immunocompetent Adult with Atypical Findings. Eur J Case Rep Intern Med 2020;7:001609. [PubMed]
- Zha J, Ma Z. Epidemiological and genetic analysis concerning the coxsackievirus A6 related endemic outbreak of hand-foot-mouth disease in Taizhou, China, during 2013. J Med Virol 2015;87:2000-8. [Crossref] [PubMed]
- Han JF, Xu S, Zhang Y, et al. Hand, foot, and mouth disease outbreak caused by coxsackievirus A6, China, 2013. J Infect 2014;69:303-5. [Crossref] [PubMed]
- Xia Y, Shan J, Ji H, et al. Study of the epidemiology and etiological characteristics of hand, foot, and mouth disease in Suzhou City, East China, 2011-2014. Arch Virol 2016;161:1933-43. [Crossref] [PubMed]
- Huang WC, Huang LM, Lu CY, et al. Atypical hand-foot-mouth disease in children: a hospital-based prospective cohort study. Virol J 2013;10:209. [Crossref] [PubMed]
- Kamau E, Nguyen D, Celma C, et al. Seroprevalence and Virologic Surveillance of Enterovirus 71 and Coxsackievirus A6, United Kingdom, 2006-2017. Emerg Infect Dis 2021;27:2261-8. [Crossref] [PubMed]
- Ju Y, Tan Z, Huang H, et al. Clinical and epidemiological characteristics of Coxsackievirus A6- and Enterovirus 71-associated clinical stage 2 and 3 severe hand, foot, and mouth disease in Guangxi, Southern China, 2017. J Infect 2020;80:121-42. [Crossref] [PubMed]
- Renert-Yuval Y, Marva E, Weil M, et al. Coxsackievirus A6 Polymorphic Exanthem in Israeli Children. Acta Derm Venereol 2016;96:546-9. [Crossref] [PubMed]
- Ji CR, Huang SQ, Xing FH, et al. Etiology and clinical characteristics of hand, foot, and mouth disease with an atypical skin rash. Journal of Parasitic Biology 2015;16:586-9.
- Li J, Zhu R, Huo D, et al. An outbreak of Coxsackievirus A6-associated hand, foot, and mouth disease in a kindergarten in Beijing in 2015. BMC Pediatr 2018;18:277. [Crossref] [PubMed]
- Aswathyraj S, Arunkumar G, Alidjinou EK, et al. Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med Microbiol Immunol 2016;205:397-407. [Crossref] [PubMed]
- Chiu HH, Liu MT, Chung WH, et al. The Mechanism of Onychomadesis (Nail Shedding) and Beau's Lines Following Hand-Foot-Mouth Disease. Viruses 2019;11:522. [Crossref] [PubMed]
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol 2000;17:7-11. [Crossref] [PubMed]
- Durán A, González A, Delgado L, et al. Serum level of C-reactive protein is not a parameter to determine the difference between viral and atypical bacterial infections. J Med Virol 2016;88:351-5. [Crossref] [PubMed]
(English Language Editor: J. Gray)


