
A comprehensive analysis of listeriosis in 13 pregnant women and 27 newborns in Xi’an from 2011 to 2020
Introduction
Listeria monocytogenes (LM), a Gram-positive bacillus, which can develop in the refrigerator over a long time and withstand large-scale temperatures (−0.4 to 45 ℃) is known as a foodborne pathogenic bacterium causing listeriosis (1). It is mainly transmitted through the consumption of contaminated food. Although cases of listeriosis are relatively rare, the mortality rate among infected humans is very high (20–30%) (2). Listeriosis causes only mild gastroenteritis in healthy individuals; however, it can cause life-threatening invasive infections in immunocompromised individuals, such as the elderly, neonates, and pregnant women (3). According to the World Health Organization, the onset of Listeria during pregnancy accounts for nearly 43% of total cases, and 14% occur in late pregnancies (4). In Europe, the incidence rates varies between 0.1 and 11.3 per million, and around 20% of the cases are perinatal or involve neonates (5). Pregnant women have an 18-fold higher risk of listeriosis compared with the general population (6). Chorioamnionitis, stillbirth, premature delivery, neonatal septicemia, meningitis, and even neonatal death are common adverse pregnancy outcomes of women infected with Listeria (7). Neonatal listeriosis can occur by direct transmission from mother to fetus. Swallowing amniotic fluid can also be a route of fetal infection (8).
Moreover, according to the recent review and meta-analysis, the published and reported cases of maternal-neonatal Listeria monocytogenes in China are still far from those in other countries and regions (9,10). In particular, there is few data about the incidence of listeriosis in newborns and pregnant women in Xi’an, with our knowledge of LM infection and its resulting disease being extremely limited. In order to find out the epidemic situation of listeriosis in newborns and pregnant women in Xi’an, to provide the basis for clinical diagnosis, treatment, corresponding prevention and control strategies for newborns and pregnant women in the local region. We retrospectively analyzed 13 maternal and 27 neonatal cases of listeriosis from January 2011 to December 2020 and a comprehensive analysis was conducted in accordance with the time of onset, clinical manifestations, laboratory indexes, treatment and outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-332/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of the Northwest Women’s and Children’s Hospital (No. 21–15). Individual consent for this retrospective study was waived. This is a retrospective study that included 40 cases of listeriosis at the Obstetrics and Neonatology Department of the Northwest Women’s and Children’s Hospital (NWCH) from January 2011 to December 2020, including 13 maternal and 27 neonatal cases. There is no generally inclusion and exclusion criteria for listeriosis. In our study, positive for isolation of LM from a normally sterile site [blood or cerebrospinal fluid (CSF)] of the pregnant women and/or newborns, were defined as confirmed cases. We collect data from the hospital’s electronic medical records including demographic characteristics, time of onset, clinical features, laboratory data, imaging examination findings, basic diseases, comorbidities, complications, treatments, inpatient days and outcomes.
Statistical analysis
The statistical analysis was conducted using SPSS version 21.0 (IBM, Armonk, NY, USA). Kolmogorov-Smirnov test was used to test the normality of the data; the normal distribution measurement data were expressed as mean ± standard deviation (SD), the non-normal distribution measurement data was expressed as the median [interquartile range (IQR)]. For categorical variables, data were provided as number (percentage).
Results
There was a total of 26,1744 pregnant women visited the obstetrics department of NWCH from 2011 to 2020. During the same period, we observed a total of 257,803 newborns including those who gave birth in NWCH and those who gave birth in an external hospital but admitted to our hospital. All the subjects were from Xi’an and its surrounding districts. Listeriosis was confirmed in 13 pregnant women and 27 newborns. Therefore, the incidence of maternal listeriosis was 5/100,000 and the ratio in newborns was 10.4/100,000 in NWCH during the past 10 years.
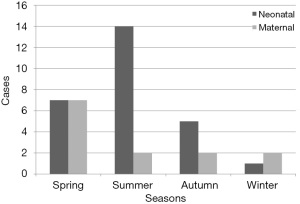
According to the season of onset, 30 cases (75%) were diagnosed with listeriosis in spring and summer. In terms of newborns, 14 cases (52%) occurred in summer and in terms of pregnant women 7 cases (54%) occurred in spring (Figure 1).
Maternal listeriosis
For the 13 hospitalized patients, the average age was (28±2.9) years and average gestation was (28.4±8.8) weeks. In terms of medical history, one patient had twin transfusion syndrome and intrahepatic cholestasis of pregnancy, while 2 patients had gestational diabetes. According to the gestational week of onset, 6 cases (46%) occurred in the second trimester (14~28 weeks), and 7 cases (54%) occurred in the third trimester (>28 weeks). Furthermore, 7 cases (54%) were cesarean section and 4 cases (31%) experienced induce abortion due to stillbirth. All of maternal listeriosis were identified by blood culture. Overall, the median length of hospital stay was 5 days (IQR, 3.5–5.5) (Table 1).
Table 1
| Characteristics | Pregnant women (n=13) | Newborns (n=27) |
|---|---|---|
| Age (years or hours) | 28±2.9 | |
| Gestation (weeks) | 28.4±8.8 | 34.5±3.5 |
| <37 (weeks) | 9 (70%) | 18 (67%) |
| 14< Gestation <28 (weeks) | 6 (46%) | 0 |
| ≥28 (weeks) | 7 (54%) | 27 (100%) |
| Birth weight (g) | 2,370±725.3 | |
| <2,500 (g) | 16 (59%) | |
| Sex | ||
| Female | 13 (100%) | 13 (48%) |
| Male | 14 (52%) | |
| Production type | ||
| Cesarean section | 7 (54%) | 20 (74%) |
| Eutocia | 2 (15%) | 7 (26%) |
| Induce abortion | 4 (31%) | |
| Basic disease | ||
| TTS and intrahepatic cholestasis | 1 (8%) | |
| Gestational diabetes | 2 (15%) | |
| Culture sites | ||
| Blood | 13 (100%) | 25 (93%) |
| Cerebrospinal fluid | 6 (22%) | |
| Gastric fluid | 1 (4%) | |
| Length of hospital stay (days) | 5 (3.5–5.5) | 21.1±15.8 |
Data are expressed as number (%), mean ± SD or median (IQR). SD, standard deviation; IQR, interquartile range; TTS, twin transfusion syndrome.
In terms of symptoms, fever (85%, n=11) and abdominal pain (77%, n=10) was the most common, followed by vaginal fluid or colporrhagia (46%, n=6), premature delivery (39%, n=5), fetal intrauterine distress (31%, n=4), premature rupture of membranes (15%, n=2) and diarrhea (8%, n=1). The histopathological results of placental tissue submitted for examination showed that 7 cases (54%) had acute chorioamnionitis (Table 2).
Table 2
| Clinical characteristics | Pregnant women (n=13) | Newborns (n=27) |
|---|---|---|
| Symptoms | ||
| Fever | 11 (85%) | 9 (33%) |
| Gastrointestinal symptoms | 11 (85%) | 1 (4%) |
| Neurological symptoms | 15 (56%) | |
| Respiratory symptoms | 14 (52%) | |
| Vaginal fluid or colporrhagia | 6 (46%) | |
| Premature delivery | 5 (39%) | 18 (67%) |
| Intrauterine distress | 4 (31%) | 14 (52%) |
| Amniotic fluid pollution | 6 (46%) | 15 (56%) |
| Premature rupture of membranes | 2 (15%) | |
| Complications | ||
| Acute chorioamnionitis | 7 (54%) | |
| Sepsis | 23 (85%) | |
| Purulent meningitis | 14 (52%) | |
| Pneumonia | 8 (30%) | |
| Septic shock | 4 (15%) | |
| Respiratory failure | 4 (15%) | |
| Incidence rate | 5/100,000 | 10.4/100,000 |
| Death ratio | 0 | 9 (33%) |
Data are expressed as number (%).
Laboratory examination showed that the level of C-reactive protein (CRP) increased most obviously (100%), followed by ratio of neutrophil counts (NEUT#) to lymphocyte counts (LYMPH#) (NLR) (92%), white blood cells (WBC) count and neutrophil counts (NEUT#) (85%), monocyte counts (MONO#) (77%). There was no obvious change in lymphocytes (LYMPH#) and procalcitonin (PCT) (Table 3).
Table 3
| Laboratory findings | Pregnant women (n=13) | Newborns (n=27) |
|---|---|---|
| WBC counts (×109/L) | 15.5±5.0 | 22.9 (12.0–29.6) |
| NEUT# (×109/L) | 12.6±4.8 | 11.8 (5.6–20.3) |
| LYMPH# (×109/L) | 1.9±0.7 | 4.9 (2.9–10.1) |
| MONO# (×109/L) | 0.9±0.4 | 2.5 (1.4–4.0) |
| NLR | 7.9±4.6 | 2.2 (1.0–5.5) |
| CRP (mg/L) | 94.6±46.7 | 61.4 (11.5–104.0) |
| PCT (ng/mL) | 0.16 (0.1–0.3) | U |
| WBC counts in CSF (×106/L) | 40.5 (17.8–782.5) | |
| Protein in CSF (g/L) | 1.4 (0.8–2.1) | |
| Glucose in CSF (mmol/L) | 1.8±1.1 |
Data are expressed as mean ± SD or median (IQR). Range of normal values: WBC, (3.5–9.5)×109/L; NEUT#, (1.8–6.3)×109/L; LYMPH#, (1.1–3.2)×109/L; MONO#, (0.1–0.6) ×109/L; NLR (0–2.8); CRP, (0–10.0) mg/L; PCT, (0–0.5) ng/mL; WBC in CSF, (0–15)×106/L; Glucose in CSF, (2.5–4.4) mmol/L; protein in CSF, (0.2–0.4) g/L. #, absolute value. SD, standard deviation; IQR, interquartile range; CRP, C-reactive protein; CSF, cerebrospinal fluid; LYMPH, lymphocyte; MONO, monocyte; NEUT, neutrophils; NLR, NEUT#/LYMPH#, PCT, procalcitonin; WBC, white blood cells.
All pregnant women received prophylactic antibiotics mainly including cephalosporins, and some were given piperacillin-tazobactam and metronidazole as supplementary treatments. After positive blood culture results were obtained, 6 cases (46%) were switched to penicillin or ampicillin and one case was switched to amoxicillin. All parturients recovered well and without sequelae. Unfortunately for their fetus, only 6 (46%) of them survived. Six of the seven dead fetuses were attributed to pregnant women in the second trimester. Of the six surviving fetuses, two were healthy at birth, and four were discharged after active treatment.
Neonatal listeriosis
For the 27 hospitalized newborns, males accounted for 52% (n=14) and females for 48% (n=13). The age range was from 0.25 hours to 8 days, with 19 (70%) of them aged less than 1 day. The average gestational age was (34.5±3.5) weeks, range from 28.3 to 39 weeks. Furthermore, they were all born in the third trimester, 67% (n=18) of which were premature (<37 weeks). The mean weight of the newborns was (2,370±725.3) g, range from 1,290 to 3,790 g and 59% (n=16) of them weighed less than 2,500 g. In the 27 culture-confirmed cases, all of which were diagnosed after positive culture in blood, CSF or gastric fluid culture, and 8 were cultured positive in more than two samples mentioned above. Overall, the median length of hospital stay was (21.1±15.8) days (IQR, 13–33.5) (Table 1).
In terms of symptoms, 89% of the cases (n=24) were born from symptomatic mothers with symptoms that including fever, abdominal pain, abnormal fetal movement, and upper respiratory tract infection, etc. Of all the newborns, about 85% (n=23) had obvious clinical symptoms, with respiratory distress was the most common (52%, n=14), followed by fever (33%, n=9). We also found that 56% of the cases (n=15) with amniotic fluid contamination. Ultimately, 23 cases (85%) showed sepsis, 14 cases (52%) showed purulent meningitis and 8 cases (30%) showed pneumonia (Table 2).
Fifty-six percent of the newborns (n=15) suffered from neurological or neurodevelopmental damage. Imaging examination findings, including B-scan ultrasonography, computed tomography, and magnetic resonance imaging, suggested intracranial hemorrhage (n=10 cases), hydrocephalus (n=3 cases), lateral ventricle widening (n=4 cases), brain injury (n=7 cases), and brain changes (n=3 cases). Screening for retinopathy of prematurity suggested incomplete retinal vascularization in both eyes in 4 cases.
Laboratory results showed that the following indicators have different degrees of increase, such as WBC count (81%), MONO# (81%), CRP (78%), NEUT# (73%), LYMPH# (73%). However, only 40% showed an increasing trend in NLR. The CSF examination revealed significantly elevated in WBC count (86%) and protein (PRO) (95%) while decreased in glucose (GLU) (73%) (Table 3).
The choice of neonatal empirical initial antibiotics was as follows: 7 cases were treated with piperacillin tazobactam alone, and 4 cases were treated with a β-lactam antibiotic (penicillin, ampicillin, or meropenem). There were 15 cases of neonates treated with cephalosporins, and 13 of them also received either penicillin or ampicillin. After the pathogen had been identified, antibiotics were specifically adjusted in 19 neonates. After active treatment, the surviving neonates accounted for 67% (n=18) and the dead accounted for 33% (n=9) of which 7 neonates’ family members voluntarily gave up on treatment or asked for discharge after knowing the prognosis, and 2 neonates died of septic shock. All the mothers of the newborns recovered well and about 41% (n=11) presented with acute chorioamnionitis.
Discussion
LM is the only pathogenic bacterium of the Listeria genus. It is widely distributed in nature and can be found in water, soil, and human and animal feces. It has been shown that about 85–90% of LM infections are caused by eating contaminated food, and the rate of bacteria in human feces is as high as 10% (11). Sporadic listeriosis has been reported to be more common during spring and summer (12), which is consistent with our results. This could be related to seasonal changes and different kinds of food, and warmer months are associated with a higher risk of infection. The findings of our study indicated that the diagnostic rate of neonatal listeriosis in summer was significantly higher than that among pregnant women. Due to the non-specificity of clinical manifestations of listeriosis during pregnancy and individual differences, clinicians fail to conduct further examination and treatment for some asymptomatic or mild pregnant women, which may be one of the reasons why some pregnant women are undiagnosed.
LM in humans is related to the quantity of bacteria and the host’s immune state. Because LM is an intracellular parasite, the host’s clearance of LM mainly depends on the cellular immune function. Therefore, the most susceptible individuals are newborns, pregnant women, adults over 40 years old, and those with immune deficiency. In high-risk populations, Listeria can cause disease even with 102 cells/g, while in healthy people it causes acute gastroenteritis with 109 cells/g (13). The amount of contaminated food consumed and the severity of contamination seem to be associated with infection, but not with the increased intrinsic virulence of specific LM strains. If there is already gastrointestinal mucosal damage caused by bacteria or viruses, LM can easily develop into an invasive disease. Listeriosis in pregnant women initially shows flu-like symptoms, fever, chills, abdominal pain, and gastrointestinal symptoms (14). As these clinical symptoms are not specific, the diagnosis is difficult.
As we know, individuals with low cellular immunity are susceptible. The increase of progesterone in pregnant women leads to a decrease in cellular immunity (15), thereby making pregnant women extremely vulnerable to infection by intracellular parasitic bacteria, such as LM. After ingestion of contaminated food, LM migrates to the mesenteric lymph nodes through the intestinal epithelial barrier, reaches the main target organs (liver and spleen), and causes infection (16). Individuals with strong immunity can effectively clear the infected foci through cellular immunity. Listeria can be released into the blood, leading to febrile bacteraemia, and eventually invasive brain infection. In pregnant women, LM is not only found in the liver and spleen but also in the uterus. Pregnant women have impaired cell-mediated immune response to LM, and with the decline of gastrointestinal motility during pregnancy, they could be prone to invasive listeriosis and subsequent transplacental infection of the fetus. Listeria has the characteristics of placental tropism. It can invade the placenta through the placental barrier and form abscesses and chorioamnionitis. In our study, about half of the cases developed acute chorioamnionitis. A recent prospective study in France highlighted that, in more than 83% of maternal listeriosis cases, there are adverse outcomes for the fetus, ranging from extremely premature birth to fetal death (17). Similarly in our study, 85% of pregnant women with listeriosis had adverse fetal outcomes and more than half of fetal deaths occurred before 29 weeks.
Neonatal infection is usually acquired in utero and occurs after delivery. Sepsis usually occurs in the early stage, and neonatal meningitis occurs 2 weeks after delivery in the late stage. In the first instance, the fetus is infected during maternal sepsis with LM or from perivaginal and perianal colonization of the mother by transition through the birth canal. Host defense against listeriosis is impaired in infants with underdeveloped macrophage and cell-mediated immune function, and invasive listeriosis is more likely to occur if colonization of the liver, respiratory tract, or gastrointestinal tract has occurred. Neonatal listeriosis can be divided into early onset (days 1–6) and late onset (days 7–28), depending on the developmental time of postnatal symptoms (18). In our study, all early-onset cases occurred in the first 24–48 hours of life. Early onset listeriosis is a characteristic of premature and seriously ill infants. In our study, 93% of the newborns had early-onset listeriosis, more than two-thirds were preterm and at least two complications coexisted, including sepsis, purulent meningitis, pneumonia, and even respiratory failure or septic shock. In the present study, the mortality of early-onset listeriosis, even with treatment, is still as high as 36% (19). Late-onset listeriosis is characterized by full-term delivery and generally born to asymptomatic mothers. Neonatal symptoms usually occur 1–2 weeks after birth, with fever and suppurative meningitis as the main manifestations, which is consistent with our results (12).
Owing to the rarity of the disease, diagnostic criteria are still lacking. Our study showed that CRP, WBC counts, NEUT#, MONO# were obviously increased in both maternal and neonatal group. It is consistent with the current study in China (20). There was no obvious change in PCT, may be affected by different blood collection time and treatment. The CSF examination showed that WBC and protein increased while glucose decreased.
Listeria is naturally resistant to cephalosporins. In our study, LM showed 100% sensitivity to ampicillin, penicillin, and pediatric compound sulfamethoxazole tablets, as well as a high sensitivity to carbapenems. The results of this study are consistent with those of Lu et al.’s (21). However, limited literature exists regarding drug resistance in the treatment of LM in pregnant females. One study reported extremely high levels of drug resistance for clindamycin (67%), penicillin G (67%), amoxicillin (50%), and vancomycin (50%) (22). Therefore, we should strengthen the monitoring of Listeria resistance through the following ways: first, all suspected Listeria infections should be sent for bacterial culture in time and drug sensitivity test should be conducted as much as possible; second, each regional laboratory regularly reports the drug sensitivity results in the national drug resistance monitoring network.
Neonatal listeriosis is one of the few congenital infections in which antibiotic treatment can improve prognosis (23). Currently, high-dose penicillin or ampicillin is the first choice for the treatment for LM infection, whereas meropenem or pediatric compound sulfamethoxazole tablets can be used for patients allergic to penicillin (24). Cephalosporins were included as the initial empirical drug treatment for all the mothers in the present study. This indicates that Listeria was not covered in the clinical treatment of puerperal infection, which could indicate that the prognosis of neonatal listeriosis is poor for mothers who have not been treated or those who have received inappropriate treatment.
The treatment time with antibiotics is generally 2 weeks from the onset of the disease to the delivery of the fetus, but the application time of antibiotics can be appropriately extended in severe cases (4). For high-risk infants, in addition to timely delivery of the fetus after termination of pregnancy, intrauterine or amniotic cavity preventive injection of antibiotics and other measures can be taken to strengthen management, minimize the probability of infection, extend gestational as much as possible, and improve the survival rate of the fetus (25). LM is the third most common cause of meningitis in neonates in the developed world, after group B Streptococci and Escherichia coli (26). The use of antibiotic prophylaxis to prevent group B streptococcal infection can also reduce the number of cases of neonatal listeriosis (27).
This study suggests that the incidence of listeriosis in pregnant women was lower than that in newborns. The possible reason was that clinicians failed to send bacterial cultures for patients with milder symptoms or remission after treatment. Unfortunately, the incidence of newborns in our hospital (10.4/100,000) was higher than that in the United States and the United Kingdom (3.4, 3.4 per 100,000 births, respectively) (28,29). This might be because our hospital is the only tertiary maternity and pediatric hospital in this area that admits many critically ill pregnant women and neonates.
Therefore, when there is unexplained fever, abdominal pain, signs of premature and respiratory symptoms accompanied by a progressive increase in WBC, CRP, NEUT#, MONO# even include WBC and PRO in CSF while GLU decreased, the possibility of an LM infection should be considered. At the same time, prenatal counseling and education to improve public awareness of the risk of foodborne diseases is important for listeriosis prevention during pregnancy. Heating or cooking food is the best way to inactivate foodborne pathogens. When it is suspected that there could be a related infection, effective treatment for Listeria should be applied, and samples should be actively sent for examination and confirmed by microbiology.
This study was a single-center retrospective study, and has some limitations, such as the number of samples included, regional differences, economic level, diet and living habits, etc. Listeriosis causes public health concern and additional large-scale studies are needed to gain a thorough understanding of this disease in China. This study summarized ten years of maternal-neonatal data in Xi’an City and it could serve as a reference for other cities and provinces of China to improve prevention, diagnosis, and treatment strategies against listeriosis.
Acknowledgments
Funding: This work was supported by the Research Project of Northwest Women’s and Children’s Hospital (program No. 2022YN02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-332/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-332/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-332/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Ethics Committee of the Northwest Women’s and Children’s Hospital (No. 21–15). Individual consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pagliano P, Arslan F, Ascione T. Epidemiology and treatment of the commonest form of listeriosis: meningitis and bacteraemia. Infez Med 2017;25:210-6. [PubMed]
- Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect 2010;16:16-23. [Crossref] [PubMed]
- Lamont RF, Sobel J, Mazaki-Tovi S, et al. Listeriosis in human pregnancy: a systematic review. J Perinat Med 2011;39:227-36. [Crossref] [PubMed]
- Wadhwa Desai R, Smith MA. Pregnancy-related listeriosis. Birth Defects Res 2017;109:324-35. [Crossref] [PubMed]
- de Noordhout CM, Devleesschauwer B, Angulo FJ, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis 2014;14:1073-82. [Crossref] [PubMed]
- Mateus T, Silva J, Maia RL, et al. Listeriosis during Pregnancy: A Public Health Concern. ISRN Obstet Gynecol 2013;2013:851712. [Crossref] [PubMed]
- Soni DK, Singh DV, Dubey SK. Pregnancy - associated human listeriosis: Virulence and genotypic analysis of Listeria monocytogenes from clinical samples. J Microbiol 2015;53:653-60. [Crossref] [PubMed]
- Li C, Zeng H, Ding X, et al. Perinatal listeriosis patients treated at a maternity hospital in Beijing, China, from 2013-2018. BMC Infect Dis 2020;20:601. [Crossref] [PubMed]
- Fan Z, Xie J, Li Y, et al. Listeriosis in mainland China: A systematic review. Int J Infect Dis 2019;81:17-24. [Crossref] [PubMed]
- Schaefer K, Austhof E, Boyd K, et al. Septicemia Due to Listeria monocytogenes Infection: A Systematic Review and Meta-Analysis. Foodborne Pathog Dis 2022;19:104-14. [Crossref] [PubMed]
- Camargo AC, Woodward JJ, Nero LA. The Continuous Challenge of Characterizing the Foodborne Pathogen Listeria monocytogenes. Foodborne Pathog Dis 2016;13:405-16. [Crossref] [PubMed]
- Schlech WF. Epidemiology and Clinical Manifestations of Listeria monocytogenes Infection. Microbiol Spectr 2019; [Crossref] [PubMed]
- Charlier C, Disson O, Lecuit M. Maternal-neonatal listeriosis. Virulence 2020;11:391-7. [Crossref] [PubMed]
- Fouks Y, Amit S, Many A, et al. Listeriosis in pregnancy: under-diagnosis despite over-treatment. J Perinatol 2018;38:26-30. [Crossref] [PubMed]
- Haidar-Ahmad N, Kissoyan KA, Fadlallah SM, et al. Genotypic and virulence characteristics of Listeria monocytogenes recovered from food items in Lebanon. J Infect Dev Ctries 2016;10:712-7. [Crossref] [PubMed]
- Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci U S A 2011;108:19484-91. [Crossref] [PubMed]
- Charlier C, Perrodeau É, Leclercq A, et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis 2017;17:510-9. [Crossref] [PubMed]
- Girard D, Leclercq A, Laurent E, et al. Pregnancy-related listeriosis in France, 1984 to 2011, with a focus on 606 cases from 1999 to 2011. Euro Surveill 2014;19:20909. [Crossref] [PubMed]
- Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect 2007;9:1236-43. [Crossref] [PubMed]
- Shi C, Lv D, Zhou K, et al. Clinical and Laboratory Characteristics of Patients infected by Listeria monocytogenes at a Tertiary Hospital in Hefei City, China. Infect Drug Resist 2021;14:4409-19. [Crossref] [PubMed]
- Lu B, Yang J, Gao C, et al. Listeriosis Cases and Genetic Diversity of Their L. monocytogenes Isolates in China, 2008-2019. Front Cell Infect Microbiol 2021;11:608352. [Crossref] [PubMed]
- Welekidan LN, Bahta YW, Teklehaimanot MG, et al. Prevalence and drug resistance pattern of Listeria monocytogenes among pregnant women in Tigray region, Northern Ethiopia: a cross-sectional study. BMC Res Notes 2019;12:538. [Crossref] [PubMed]
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in Pregnancy: A Review. Obstet Gynecol Surv 2019;74:362-8. [Crossref] [PubMed]
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;39:1267-84. [Crossref] [PubMed]
- Bubonja-Sonje M, Mustac E, Brunn A, et al. Listeriosis in pregnancy: case report and retrospective study. J Matern Fetal Neonatal Med 2013;26:321-3. [Crossref] [PubMed]
- Okike IO, Johnson AP, Henderson KL, et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis 2014;59:e150-7. [Crossref] [PubMed]
- Baltimore RS, Huie SM, Meek JI, et al. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics 2001;108:1094-8. [Crossref] [PubMed]
- Sapuan S, Kortsalioudaki C, Anthony M, et al. Neonatal listeriosis in the UK 2004–2014. J Infect 2017;74:236-42. [Crossref] [PubMed]
- Jeffs E, Williman J, Brunton C, et al. The epidemiology of listeriosis in pregnant women and children in New Zealand from 1997 to 2016: an observational study. BMC Public Health 2020;20:116. [Crossref] [PubMed]


