Visuoperceptual sequelae in children with hemophilia and intracranial hemorrhage
Introduction
There has been great debate in the field of developmental neuropsychology during the last century regarding the concept of brain plasticity in children with early brain injury. Early plasticity theorists have stipulated that a young brain is immature and as a consequence is less susceptible to the impact of cerebral damage (1-4). Conversely, early vulnerability theorists have proposed that brain injury sustained during the developmental window results in significant long-term neuropsychological impairment (5-8). There are many different factors to be considered in this debate. First, plasticity has been demonstrated in both animal models and human studies (9-12). Second, early vulnerability is based on studies that documented impairments in abilities that are relevant to the acquisition of cognitive processes and skills (7,13-15). Finally, neuropsychological outcomes in both theories are dependent upon different variables such as the type, severity, and age of onset of a lesion, environmental factors, and developmental parameters (10,14,16,17). While there has been a great deal of research to support the contrasting sides of this debate, further exploration is needed to elucidate how early plasticity and early vulnerability theories actually apply in different clinical circumstances. In the field of neuropsychology, brain damage is studied using the nature and severity of the lesion as variables. Generalized brain trauma and focal brain lesions are the two most commonly studied lesions in plasticity and vulnerability theories. In general, when a lesion is generalized, poor outcomes and global cerebral dysfunction are expected. For example, in traumatic brain injuries where damage is diffused, functional deficits are seen in many cognitive areas, such as attention, memory and learning, psychomotor skills, linguistic abilities, and executive functions (13). In contrast, focal brain lesions are characterized by moderate-to-good outcomes in most cognitive domains, but some deficits have been noted in particular areas (7,18).
The understanding of developmental plasticity and recovery from injury comes from studies of focal brain lesions experienced at particular developmental stages. Furthermore, other cognitive domains are often impaired by early focal brain injury. For example, studies have found that children with early focal lesions manifest difficulties later on in development of higher-level verbal, visual-spatial executive, and information-processing abilities (5,18,19). These findings suggest a variety of factors that interact with focal brain injury to impact specific cognitive outcomes such as attention, memory, and executive functioning, among others.
A growing body of theoretical and empirical work on visual perception areas such as visual recognition, visual organization, and visual interference areas in adults suggests that visual perception may be affected in adulthood after focal brain damage occurs in childhood (20-22). Early trauma or other forms of acquired injury often affect substantial portions of the brain, resulting in damage that could compromise cognitive ability in adulthood. In the specific case of focal brain injury, literature suggests that affected children are at high risk of suffering visual perception and visuospatial deficits, since the development of the neural networks subserving these cognitive functions does not conclude until late adolescence (23). Moreover, the basic neural system that mediates part—whole processing may begin to develop early and continues to undergo significant developmental change for a continued period of time (23-25). Studies addressing specific visual perception and visuospatial abilities in children with focal brain injuries are limited, however.
Cerebral hemorrhage in children with hemophilia
One of the most severe and devastating manifestations in patients with clotting disorders is intracranial hemorrhage, which directly affects the central nervous system. Hemophilia is a clotting disease that can cause intracranial hemorrhage due to deficiencies in coagulation factors VIII and IX. Children with hemophilia who have experienced intracranial hemorrhages often received hemorrhages at the time of birth or during the first 2 years of life (26). The literature suggests that children with hemophilia and intracranial hemorrhage show lower performance in visuospatial skills than children from a healthy control group (27,28) and have worse clinical outcomes, especially in the domains of quality of life (29), motor function, visual-motor integration, and behavior (27). Some studies examining neuropsychological outcomes in children with hemophilia who have experienced intracranial hemorrhage have shown a correlation between low visual-motor integration and reduced intelligence, compared with normal controls (27-31). Although some cognitive domains seem to confirm damage in children with hemophilia who have experienced intracranial hemorrhage, the question remains: is there evidence that damage affects visual perception and visuospatial abilities in children with hemophilia and intracranial hemorrhage?
The limited data available that address visual perception and visuospatial abilities in children with hemophilia are not sufficient enough to establish a causal relationship between the illness and these cognitive deficits. Furthermore, because the studies addressing these areas in children with focal brain injuries are limited, conclusions covering these subjects cannot be drawn at this time. The goal of this study is to examine the impact of intracranial hemorrhage on visual perception and visuospatial abilities, as well as outcomes in Mexican children with hemophilia who have experienced intracranial hemorrhage. We hypothesized that children with intracranial hemorrhages will have lower performance in visual perception and visuospatial abilities compared with children with hemophilia who do not have a history of intracranial hemorrhage and to matched controls.
Materials and methods
Participants
The participants in this study were drawn from a larger sample of 118 children with hemophilia from the Children’s Hospital at the Mexican Institute of Social Security in Guadalajara, Mexico. The children with hemophilia were identified through records listing all patients with hemophilia A or B who had received treatment at the Hematology/Oncology Department. Children with hemophilia with intracranial hemorrhage (HIC), children with hemophilia without intracranial hemorrhage (HH), and normal controls (CLT) were included in this study. For the total of children participating in this study, the following inclusion criteria were applied: (I) the ability to complete a cognitive evaluation; (II) age from 5 to 16 years at the time of assessment; and (II) belong to the Mexican Social Security Institute at Guadalajara. The exclusion criteria were (I) psychiatric history of psychotic disorders; (II) positive human immunodeficiency virus status; and (III) living a great distance away from our testing center. The inclusion criteria for the intracranial hemorrhage group was (I) previous cerebral hemorrhage(s) documented by head computed tomography. The inclusion criteria for the hemophilia group was (I) no history of previous cerebral hemorrhage, and (II) a magnetic resonance image conducted within one week prior to the neuropsychological assessment to verify that they did not have evidence of old bleeds or brain injury. Details of the sample and recruitment strategies have been described previously (32). Each child with hemophilia and intracranial hemorrhages was matched case by case with a boy with hemophilia without intracranial hemorrhage and a healthy boy who attended the same classroom, lived in the same neighborhood, was of the same socioeconomic status (SES), and was the closest in date of birth.Previous brain lesions in the HIC group were identified through a review of clinical charts and notes. The lateralization of the lesion and its localization in each of the four lobes (frontal, parietal, temporal, and occipital) were also identified through chart review. In addition, the number of intracranial hemorrhagic events was identified (see Table 1 for intracranial hemorrhage characteristics).
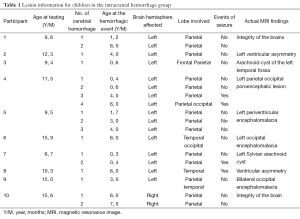
Full table
All of the participants were screened (via parent or legal guardian interviews) for severe developmental disorders, psychiatric history, and human immunodeficiency virus status. None of the identified participants were in full-time special education, so no one was excluded on the basis of these criteria. SES was determined using the Socioeconomic Stratification of Metropolitan Zone of Guadalajara (33) which rates the observable distinctions of housing, public services, and type of neighborhood into high, middle, low, and marginal socioeconomic levels. Among the participants, the majority were from low or marginal socioeconomic levels. Demographic characteristics of the sample are provided in Table 2.
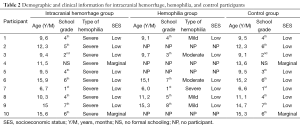
Full table
Procedures
This study was approved by the Human Research Ethics Committee of the Children’s Hospital and Ethics Committee of the Institute of Neurosciences in Guadalajara, Mexico. Written, informed consent was obtained from all participants and their parents or legal guardians prior to the study. Testing was completed at a time and location convenient for each family (most commonly in the home) by a trained graduate student in neuropsychology who was blinded to the clinical status of the participants. The families were compensated with a box of groceries for their participation in this phase of the study.
A magnetic resonance imaging (MRI) study was performed for the HIC and HH groups to verify evidence of new bleeds or brain injuries prior to the neuropsychological assessment. All MRI images were independently reviewed by two experienced neuroradiologists who were blind to the clinical status of the participants. Table 3 includes a list of neuropsychological tests used in the assessment as well as a description of the domains assessed.
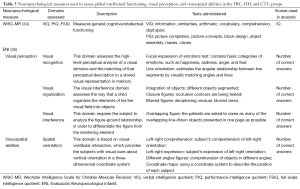
Full table
Statistical analysis
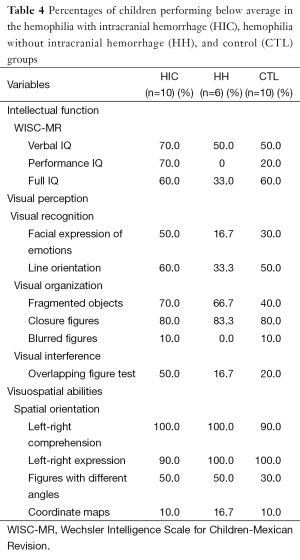
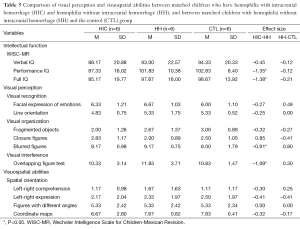
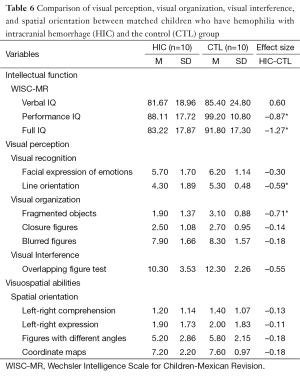
The data were examined in three different ways. First, the percentages of children performing below average were tabulated for each scale within each group (Table 4). Then, Cohen’s d (effect size) was calculated to reflect the difference between the means of each pair of groups (HIC-HH, HH-CTL, and HIC-CTL) for each scale (Tables 5,6). Lastly, each pair of groups was compared on each of the scales using the Wilcoxon matched-pairs signed-ranks test (Tables 5,6) because the data were paired data, not independent observations. The participants were matched across groups. Consequently, when comparisons were made with the HH group, only six participants could be included in the analysis. When the HIC and CTL groups were compared, there were ten participants in each group. It would not have been appropriate to use the chi-square test or Fisher’s exact test for two reasons: (I) these tests require independent observations; and (II) these tests require frequency data. Neither of these requirements was met in the current data. Using a statistical test substantially increases the power of paired data, which is especially important in a small sample. Also, Cohen’s d was used purely as a descriptive statistic. The assumptions for the parametric, dependent t-test did not need to be met because no parametric test was run.
Full table
Full table
Full table
Results
From these 118 children with hemophilia, 102 children could not be recruited for the study due to these study criteria: long geographic distances (n=2), a psychiatric history of psychotic disorders (n=17), human immunodeficiency virus positive status (n=11), not currently belonging to the Mexican Social Security Institute (n=59), or refusal to participate in the neuropsychological examination (n=13). The remaining 16 boys with hemophilia were selected for our study. Only 10 children with hemophilia who had experienced intracranial hemorrhages (HIC group) were identified; their ages ranged between 6 and 15 years (X=11.10; SD =3.10). In order to have much better control of the variables, authors decided to pair the ten HIC children with HH children. Due to the specific characteristics of the HIC group, it was difficult to find ten children with hemophilia without intracranial hemorrhage (HH group) with the same criteria. We paired the HIC group with six children with hemophilia but without intracranial hemorrhages between 6 and 15 years old (X=10.83; SD =3.60). A control group of 10 healthy children between 6 and 15 years old (X=11.30; SD =3.02) was also included in this study.
All patients with hemophilia included in this study were diagnosed with hemophilia A. The severity of the hemophilia in the sample was as follows: 68.7% of children with hemophilia had severe hemophilia (defined as <1% clotting factor), 12.6% had moderate hemophilia (1% to 5% clotting factor), and 18.7% had mild hemophilia (>5% clotting factor).
The percentage of children performing below average was highest in the HIC group for five of the scales (PIQ, overlapping figures, and facial expression of emotions, fragmented objects, and line orientation). When comparing the HH and CTL groups, the percentage was higher for the HH group than the CTL group on five scales (fragmented objects, closure figures, left-right comprehension, different angle figures, and coordinate maps), lower on four other scales (facial expressions of emotions, line orientation, blurred figures, and overlapping figures), and equal on one scale (left-right expression) (see Table 4).
In the comparison of the HIC and HH groups, the largest Cohen’s d (d=0.2 as a small effect size, d=0.5 as a medium effect size, and d =0.8 as a large effect size) were found for the WISC-MR: Full IQ (d=–1.38) and Performance IQ (d=–1.35). The ENI showed the largest Cohen’s d in visual interference area for overlapping figures (d=–1.09); the next largest were in the area of visual organization for blurred figures (d=–0.91) and closure figures (d=0.85). For the comparison of the HH and CTL groups, the WISC-MR showed the largest Cohen’s d for Full IQ (d=1.27), and the next largest was for Performance IQ (d=–0.87). In the ENI subscales, the Cohen’s ds were generally quite small, the largest being found in the visual organization area for blurred figures (d=0.80) (see Table 5). In the comparison of the HIC and CTL groups, the largest Cohen’s d was found in the visual organization area for fragmented objects (d=–0.71), followed by the visual recognition area for line orientation (d=–0.59) and visual interference area for overlapping figures (d=–0.55). Nearly all ds were in the expected and the CTL group (see Table 6).
For the Wilcoxon matched-pairs signed-ranks tests, P was set at 0.05 to allow for maximum sensitivity in this small sample. These tests produced significant differences for two scales in the comparison between HIC and HH, no scales in the comparison between HH and CTL, and two scales in the comparison between HIC and CTL. HIC and HH differed significantly on PIQ, FSIQ, overlapping figures, and blurred figures. These differences reflected poorer performance by the HIC group. HIC and CTL differed significantly on PIQ, FSIQ, integration of objects, and line orientation. Again, the differences reflected poorer performance by the HIC group (see Tables 5,6).
Discussion
This study examined the impact of focal brain injury on visual perception and visuospatial abilities in Mexican children with hemophilia who had intracranial hemorrhages. We hypothesized that children with hemophilia who experienced intracranial hemorrhage would have lower performance in visual perception and visuospatial abilities than children with hemophilia who did not experience intracranial hemorrhage and their matched controls. The results were as predicted in the visual perception area, the HIC group showed low performance on visual perception tests, such as line orientation, fragmented objects, and overlapping figures, compared with their matched controls. However, visuospatial abilities were not significantly different among the three groups.
Effects of intracranial hemorrhage
This study found that children with hemophilia and intracranial hemorrhage showed PIQ and FSIQ scores in the low-to-average range. This result is consistent with results reported by previous studies which used the same methodology (age at lesion, age at testing, and number of strokes) and IQ materials (21,22). These studies have shown that children who had experienced focal brain injury achieved IQ scores in the low-average to average range (21,22). Moreover, earlier brain lesions are associated with lower intellectual quotient and intellectual quotient deterioration over time (21). Our research supported these findings and found that early brain damage influences cognitive outcomes in children with hemophilia who have suffered intracranial hemorrhage. Additionally, members of the HIC group showed difficulties with visual perception areas, such as visual recognition, visual organization, and visual interference tasks, compared with their matched controls. Visual recognition tasks are used to assess visual perception, and in these tasks, the child must have the ability to interpret facial expressions and other nonverbal cues. This ability plays an important role in the development and maintenance of intimate human relationships (36).
In our study, the HIC group performed significantly worse than the control group on the line orientation task. The majority of children in the HIC group scored below average in this task compared with the HH and CTL groups. Previous research supports our findings and has also found that individuals who have experienced brain damage (37,38) or genetic disorders such as neurofibromatosistype-1 (39) and Turner syndrome (40) typically have significant difficulties with this line orientation task. Face recognition tasks did not show significant variances between the three groups; however, the HIC group performed below average on this task. In the case of visual organization, the HIC group’s performance was lower than the HH group’s performance in the integration of objects and blurred figures. These tasks primarily focus on visual recognition of objects from partial or degraded images resulting from viewing objects from different viewpoints, which often causes features of the object to be blocked, cut up, or altered (41). One purpose for the use of these tests is to identify the possibility of object recognition breakdowns, which may cause individuals to lose stored knowledge about objects. Without stored knowledge, object recognition cannot occur. Finally, the HIC group demonstrated low performance on the visual interference task compared with the HH and CTL groups. On this task, the HIC group had significant difficulty with identifying parts of the visually presented object and understanding how the parts combined to form a unified whole.
Overall, the results of the present study revealed differences between the HIC and HH groups for PIQ and FIQ and some visual perception tasks. However, no differences were found between the HH and CTL groups. In other words, the differences that were found in this study were exclusively related to intracranial hemorrhage. This finding is consistent with the literature, as different studies have found lower neuropsychological performance in children with hemophilia who presented a history of intracranial hemorrhage (27-29,42).
Effects of hemophilia
One unexpected finding of our study was that performance was not significantly different between the HH and CTL groups. This result is not consistent with the literature due to the potential negative impact of hemophilia on academic and cognitive functioning suggested by previous studies (43,44). The similar performance of the HH and CTL groups further indicates that these groups are not likely to be carrying out tasks in different manners or using different strategies to recognize objects. In this sample, both the HH and CTL groups were matched on SES, and members of both the HH and CTL groups grew up in low-SES environments. Apart from other cultural contexts, support from family is particularly important for Mexicans with chronic illnesses such as hemophilia. In this regard, parents and families are protective of their children, and this protection increases during times of illness. Ultimately, protective factors such as these may have influenced the performance of those in the HH group on these tasks.
In this context, it is important to mention that the severity of hemophilia is an important variable to be analyzed. Our study included 11 participants with severe hemophilia (10 participants in the HIC group and 1 in the HH group), 3 participants with mild hemophilia (belonging to the HH group), and 2 participants with moderate hemophilia (belonging to the HH group). In order to analyze this variable, we would need a greater sample size to group them according to severity. Although the literature has stated that patients with severe hemophilia suffer complications such as bleeding episodes and such bleeding can affect the central nervous system (27-29,42), different studies have suggested that hemophilia severity is not significantly related to neuropsychological outcomes (45,46).
Visuospatial abilities
In our study, the three groups did not show statistically different performance in terms of visuospatial abilities. It appears that all three groups showed difficulties in this area, as the majority of the participants had below-average abilities in left-right comprehension and expression tasks. It is important to highlight that children typically apply these terms to their own bodies correctly around seven years of age, in addition to applying them to another person facing them (47). The use and determination of a person’s position with reference to right and left wording evolves as age increases, and the steps in verbal use of right and left terms are well known. The evolution of mental representation is not age dependent; however, the evolution is dependent on cognitive development. In part, this is because we know that development is gradual and cannot take place from 1 year to the next (48). Although left-right comprehension and expression are dependent on cognitive development rather than being age dependent, this process may be delayed due to environmental factors, such as lower SES level, deferred school years, and chronic illness.
Clinical implications
Our findings have important clinical implications for the use of neuropsychological assessments in high-risk children with hemophilia and children with hemophilia who have experienced intracranial hemorrhage. The impacts of problems with visual perception and visuospatial functioning on the everyday lives of children emphasize the importance of early detection of visual functioning difficulties and relevant interventions for these children. Therefore, it is not possible to know the potential academic impact of visual perception and visual spatial deficits in children with hemophilia. However, the importance of visual perception and visuospatial abilities to academic achievement in such areas as reading and math is well established in children without chronic illness (49). As a result, it is likely that children with hemophilia who have histories of intracranial hemorrhage and visual perception and/or visuospatial difficulties are at risk for academic challenges. Among neuropsychology studies, hemophilia is a genetic disorder that is not well understood. Studies of patients with hemophilia have mainly focused on outcomes associated with HIV status, behavior, and social interactions. The majority of those results were derived from the Hemophilia Growth and Development Study (50). We are not aware of any studies that have examined visual perception and visuospatial abilities in children with hemophilia, which makes this topic of greater interest and provides an opportunity for future explorations in this area.
Limitations and future directions
A variety of potential limitations should be considered when interpreting the results of our study. The small sample size in each group limited the power of the group comparisons to detect the effects of intracranial hemorrhage. Next, the participants in this study varied substantially in age. Finally, given the wide variation in lesion locations and numbers of intracranial hemorrhages, a large sample would provide more rigorous results of the moderating influences of these factors. In future studies, it would prove beneficial to examine visual perception and visuospatial abilities in a group of children with hemophilia who had intracranial hemorrhages within a smaller age range in order to consider more precise questions regarding the effects of development on visual perception and visuospatial performance.
While unanswered questions remain for future research efforts, hematology practices can now incorporate routine screening via regular neuropsychological assessments of the visual perception and visuospatial abilities discussed in this work. Specifically, neuropsychologists working within hematology clinics can recognize the importance of neuropsychological evaluations that include visual perception (visual recognition, visual organization, and visual interference) and visuospatial abilities (visual construction, visuospatial orientation, and spatial visualization). This study can serve as a pilot study for the remainder of patients with hemophilia in Latin America, in order to ascertain whether Mexican patients with hemophilia display specific cultural characteristics that must be taken into account, especially when considering the impact of hemophilia on individual development.
Acknowledgements
We express our special thanks to Dr. Arthur Partikian, director of the Division of Child Neurology at LAC+USC Medical Center, Los Angeles, California, for his thoughtful observations on this manuscript. We gratefully acknowledge Dr. Ana Rebeca Jaloma-Cruz and Dr. María Amparo Esparza-Flores for their assistance with participant recruitment and Jorge Arceo-Morales for helping with participant transportation.
Funding: This research was supported in part by a grant: Molecular diagnosis in patients and carriers of hemophilia A (2000-03-02-021) from Consejo Nacional de Ciencia y Tecnología; by University of Guadalajara, Neuroscience Institute; by a gift of Centro de Diagnostico Rio; and by a gift of El Palomar Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Burgess JW, Villablanca JR. Recovery of function after neonatal or adult hemispherectomy in cats. II. Limb bias and development, paw usage, locomotion and rehabilitative effects of exercise. Behav Brain Res 1986;20:1-17. [PubMed]
- Kennard MA. Cortical reorganization of motor function studies on series of monkeys of various ages from infancy to maturity. Arch NeurPsych 1942;48:227-40.
- Kolb B, Tomie JA. Recovery from early cortical damage in rats. IV. Effects of hemidecortication at 1, 5 or 10 days of age on cerebral anatomy and behavior. Behav Brain Res 1988;28:259-74. [PubMed]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 1970;206:419-36. [PubMed]
- Anderson V, Spencer-Smith M, Coleman L, et al. Children's executive functions: are they poorer after very early brain insult. Neuropsychologia 2010;48:2041-50. [PubMed]
- Anderson V, Jacobs R, Spencer-Smith M, et al. Does early age at brain insult predict worse outcome? Neuropsychological implications. J Pediatr Psychol 2010;35:716-27. [PubMed]
- Max JE. Effect of side of lesion on neuropsychological performance in childhood stroke. J Int Neuropsychol Soc 2004;10:698-708. [PubMed]
- Stiles J, Reilly J, Paul B, et al. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci 2005;9:136-43. [PubMed]
- Dennis M. Developmental plasticity in children: the role of biological risk, development, time, and reserve. J Commun Disord 2000;33:321-31. [PubMed]
- Giza CC, Prins ML. Is being plastic fantastic? Mechanisms of altered plasticity after developmental traumatic brain injury. Dev Neurosci 2006;28:364-79. [PubMed]
- Stiles J. Neural plasticity and cognitive development. Dev Neuropsychol 2000;18:237-72. [PubMed]
- Stiles J, Reilly J, Paul B, et al. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci 2005;9:136-43. [PubMed]
- Anderson V, Spencer-Smith M, Leventer R, et al. Childhood brain insult: can age at insult help us predict outcome? Brain 2009;132:45-56. [PubMed]
- Chapman SB, Max JE, Gamino JF, et al. Discourse plasticity in children after stroke: age at injury and lesion effects. Pediatr Neurol 2003;29:34-41. [PubMed]
- Ewing-Cobbs L, Fletcher JM, Levin HS, et al. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc 1997;3:581-91. [PubMed]
- Anderson V, Northam E, Hendy J, et al. eds. Developmental Neuropsychology: A clinical Approach. Hove & New York: Taylor & Francis Psychology Press, 2001.
- Vicari S, Albertoni A, Chilosi AM, et al. Plasticity and reorganization during language development in children with early brain injury. Cortex 2000;36:31-46. [PubMed]
- Everts R, Pavlovic J, Kaufmann F, et al. Cognitive functioning, behavior, and quality of life after stroke in childhood. Child Neuropsychol 2008;14:323-38. [PubMed]
- Stiles J, Stern C, Appelbaum M, et al. Effects of early focal brain injury on memory for visuospatial patterns: selective deficits of global-local processing. Neuropsychology 2008;22:61-73. [PubMed]
- Selzer SC, Lindgren SD, Blackman JA. Long-term neuropsychological outcome of high risk infants with intracranial hemorrhage. J Pediatr Psychol 1992;17:407-22. [PubMed]
- Duval J, Braun CM, Montour-Proulx I, et al. Brain lesions and IQ: recovery versus decline depends on age of onset. J Child Neurol 2008;23:663-8. [PubMed]
- Hogan AM, Kirkham FJ, Isaacs EB. Intelligence after stroke in childhood: review of the literature and suggestions for future research. J Child Neurol 2000;15:325-32. [PubMed]
- Stiles J, Stern C, Trauner D, et al. Developmental change in spatial grouping activity among children with early focal brain injury: evidence from a modeling task. Brain Cogn 1996;31:46-62. [PubMed]
- Stiles J, Moses P, Roe K, et al. Alternative brain organization after prenatal cerebral injury: convergent fMRI and cognitive data. J Int Neuropsychol Soc 2003;9:604-22. [PubMed]
- Stiers P, De Cock P, Vandenbussche E. Separating visual perception and non-verbal intelligence in children with early brain injury. Brain Dev 1999;21:397-406. [PubMed]
- Klinge J, Auberger K, Auerswald G, et al. Prevalence and outcome of intracranial haemorrhage in haemophiliacs--a survey of the paediatric group of the German Society of Thrombosis and Haemostasis (GTH). Eur J Pediatr 1999;158 Suppl 3:S162-5. [PubMed]
- Bladen M, Khair K, Liesner R, et al. Long-term consequences of intracranial haemorrhage in children with haemophilia. Haemophilia 2009;15:184-92. [PubMed]
- Miles BS, Anderson P, Agostino A, et al. Effect of intracranial bleeds on the neurocognitive, academic, behavioural and adaptive functioning of boys with haemophilia. Haemophilia 2012;18:229-34. [PubMed]
- Revel-Vilk S, Golomb MR, Achonu C, et al. Effect of intracranial bleeds on the health and quality of life of boys with hemophilia. J Pediatr 2004;144:490-5. [PubMed]
- Basu J, Chowdhury MR, Mitra AK. Cognitive functioning, personality variables and academic achievement of hemophiliac and normal children: a comparative study. Psychol Stud 2010;55:165-71.
- Watkins JM, Cool VA, Usner D, et al. Attention in HIV-infected children: results from the Hemophilia Growth and Development Study. J Int Neuropsychol Soc 2000;6:443-54. [PubMed]
- Morales G, Matute E, Murray J, et al. Is executive function intact after pediatric intracranial hemorrhage? A sample of Mexican children with hemophilia. Clin Pediatr (Phila) 2013;52:950-9. [PubMed]
- Instituto Nacional de Estadística, Geografía e Informática. Indicadores sociodemográficos de México (1930-2000). Available online: http://www.inegi.org.mx/prod_serv/contenidos/espanol/bvinegi/productos/integracion/sociodemografico/indisociodem/2001/indi2001.pdf. Accessed February 20, 2014.
- Wechsler D. Manual de administración y calificación de la Escala de Inteligencia Wechsler para niños, revisión Mexicana [Administration and scoring manual for the Wechsler Intelligence Scale for Children, Mexican Revision]. México City, México: Manual Moderno, 1984.
- Matute E, Rosselli M, Ardila A, et al. ENI: Evaluación Neuropsicológica Infantil [Child Neuropsychological Evaluation]. Guadalajara, México: Manual Moderno-UNAM-Universidad de Guadalajara, 2007.
- Matsumoto D, Keltner D, Shiota M et al. Facial expression of emotion. In: Lewis M, Haviland-Jones JM, Barret LF. eds. Handbook of Emotions, Third Edition. New York, NY: Guildford Press, 2010:211-324.
- Bogdanova Y, Neargarder S, Cronin-Golomb A. Mapping mental number line in physical space: vertical and horizontal visual number line orientation in asymptomatic individuals with HIV. Neuropsychologia 2008;46:2914-23. [PubMed]
- van den Hout BM, de Vries LS, Meiners LC, et al. Visual perceptual impairment in children at 5 years of age with perinatal haemorrhagic or ischaemic brain damage in relation to cerebral magnetic resonance imaging. Brain Dev 2004;26:251-61. [PubMed]
- Clements-Stephens AM, Rimrodt SL, Gaur P, et al. Visuospatial processing in children with neurofibromatosis type 1. Neuropsychologia 2008;46:690-7. [PubMed]
- Kesler SR, Haberecht MF, Menon V, et al. Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cereb Cortex 2004;14:174-80. [PubMed]
- Riesenhuber M, Poggio T. Models of object recognition. Nat Neurosci 2000;3 Suppl:1199-204. [PubMed]
- Nuss R, Soucie JM, Evatt B, et al. Changes in the occurrence of and risk factors for hemophilia-associated intracranial hemorrhage. Am J Hematol 2001;68:37-42. [PubMed]
- Sexson S, Madan-Swain A. The chronically ill child in the school. School Psychol Quart 1995;10:359-68.
- Woolf A, Rappaport L, Reardon P, et al. School functioning and disease severity in boys with hemophilia. J Dev Behav Pediatr 1989;10:81-5. [PubMed]
- Sirois PA, Usner DW, Hill SD, et al. Hemophilia growth and development study: relationships between neuropsychological, neurological, and MRI findings at baseline. J Pediatr Psychol 1998;23:45-56. [PubMed]
- Usner DW, Donfield SM, Sirois PA, et al. Hemophilia morbidity, cognitive functioning, and academic achievement. J Pediatr 1998;133:782-7. [PubMed]
- Piaget J. eds. The mechanism of perception. Cook M, trans. New York: Basic Books, 1969.
- Regal DM. Development of Critical flicker frequency in human infants. Vision Res 1981;21:549-55. [PubMed]
- Dessalegn B, Landau B, Rapp B. Consequences of severe visual-spatial deficits for reading acquisition: evidence from Williams syndrome. Neurocase 2013;19:328-47. [PubMed]
- Hilgartner MW, Donfield SM, Willoughby A, et al. Hemophilia growth and development study. Design, methods, and entry data. Am J Pediatr Hematol Oncol 1993;15:208-18. [PubMed]


