Intracranial hemorrhage associated with late-onset group B streptococcus disease—a case report and a review of literature
Introduction
Group B streptococcus (GBS) or streptococcus agalactiae, is a most common cause of infection during pregnancy, preterm birth and neonatal infection. GBS is a leading factor to adverse maternal and newborn outcomes. Late-onset disease (LOD) occurs from day 7 to 89. The LOD incidence based on population in the literature varies. In a global meta-analysis (1), the average incidence of LOD was 0.26 per 1,000 live births (95% CI: 0.21–0.30). The source of LOD infection remains unclear. The infection may be the result of horizontal transmission (nosocomial acquisition) and the colonization of mothers. The most common clinical syndromes of LOD were sepsis, meningitis and focal infection (cellulitis, osteomyelitis, or septic arthritis) (2).
Here we present a case that was admitted to the hospital with fever and respiratory arrest, who did not get vaccination on time and the mother had a history of mastitis; the diagnosis was intracranial hemorrhage caused by late-onset GBS infection.
Case presentation
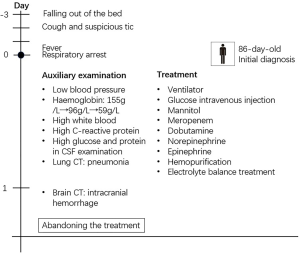
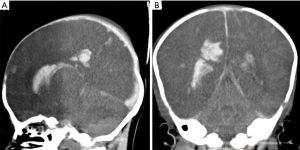
A male baby weighing 3.6 kg was born as a full-term baby via caesarean section with no special disease during pregnancy. He was well-being until 83 days of age when he fell out of the bed. After the onset of falling, he became cough and had suspicious tic within 2 days and fever was noticed within half a day. The patient was hospitalized because of respiratory arrest with cannula. On admission, the patient’s weight was 7.6 kg, temperature was 39.2 °C and pulse was 200 times/min with apnea and unclear blood pressure. High tension in bregma was noted. The baby did not undergo prophylactic vaccination on time. The baby’s mother recently has an illness history of mastitis. Later, physical and auxiliary examinations were done. The blood pressure was 43/28 mmHg. Hemoglobin was 156 g/L at the first examination, and dropped down to 94 g/L after 3 hours and 59 g/L after 9 hours. White blood cell count was 2.54×109/L, the percentage of neutrophil granulocyte was 34% and C-reactive protein was 19.54 mg/L. The blood sugar was 2.1 mmol/L on admission. In cerebrospinal fluid (CSF) examination, glucose was 0.42 mmol/L, protein was 3,069 mg/L. GBS was positive in the CSF and blood culture. The diagnosis was late-onset neonatal sepsis and purulent meningitis caused by GBS infection. Although the patient had a poor condition and it was risky to go out for a computed tomography (CT), we arranged CT with the agreement of patients. The result of CT of the brain showed intracranial hemorrhage and cerebral swelling. The possible diagnoses included intraventricular hemorrhage (IVH), subarachnoid hemorrhage and hypoxic-ischemic encephalopathy (HIE) and the hemorrhage caused by trauma was excluded (Figures 1,2).
Respiratory support was immediately started after the admission. After the discovery of low blood glucose and increased intracranial pressure, the patient was immediately given glucose intravenous injection and mannitol to alleviate the pressure. The treatment with meropenem, dobutamine, norepinephrine and epinephrine were started. The patient was injected with cryoprecipitate and human immunoglobulin. The patient also was managed with hemopurification and electrolyte balance treatment.
The patient was still in severe conditions. GBS infection led to meningitis, intracranial hemorrhage and sepsis. Other complications included multiple organ dysfunction syndrome (MODS), fluid and electrolyte imbalance, disseminated intravascular coagulation (DIC), bronchopneumonia and cerebral hernia. The patient had no autonomous respiration and had sinus rhythm for many times. During the treatment, the patient suffered cardiac arrest repeatedly. It was risky to operate the surgery to alleviate the intracranial pressure. What’s more, the patient had severe sepsis, leading to dysfunctional circulatory system. Taking repeated cardiac arrest in to consideration, the patient may suffer from heart failure. The intracranial hemorrhage may also lead to irreversible damage in brain. Despite active and proper treatment in time, the patient’s parents required to abandon the treatments considering the severe condition and poor prognosis.
Discussion
GBS is a leading factor to adverse maternal and newborn outcomes. Late-onset GBS infection can be characterized by various syndromes in clinical. Several complications have been reported in cases, including sepsis, meningitis, HIE, pneumonia and cerebral edema (3-5), but intracranial hemorrhage is rarely reported. GBS can cross the blood-brain barrier and cause meningitis. GBS can invade the brain through transcellular route and also invade brain endothelial cells through a serious of surface protein. Occurrence of meningitis may have an influence on the onset of intracranial hemorrhage, considering the increased intracranial pressure and inflammation.
The pathogenesis of LOD is not as well understood as that of early-onset disease (EOD). Prematurity is the most important risk factor for LOD (6). Also, a mother of black race (7) and exposure to HIV (8) are risk factors for LOD. The GBS colonization of mother (genital and/or lower gastrointestinal tract) plays a role in LOD, too.
Based on the former cases report of LOD, the infection is likely result from the nosocomial acquisition or contaminated breast milk.
While breast milk can protect infants from infection to some extent, case reports have implicated that transmission of GBS through breast milk as a possible cause of LOD. GBS contaminated breast milk is associated with heavy neonatal colonization. In our case, the mother had the history of mastitis. It meant higher level of bacterial GBS counts, which may lead to neonatal colonization.
One possible mechanism of the contaminated breast milk is through retrograde transmission (2). The colonization of GBS in infants’ oral mucosal contaminates the maternal mammary ducts. It multiplies in the mammary ducts and re-colonizes in infants. Another possible mechanism is the translocation of infant-derived GBS from the gastrointestinal tract to the mammary glands (2).
In our case, the baby was born on mixed feeding. The mother had a history of mastitis. This may be a possible cause of GBS infection. However, a test on the patient’s mother have not been down, so we could not know what was the specific infection resource. Infants who were often fed with a combination of formula and fortifier may suffer intestinal mucosa damage and be more susceptible to infection from breast milk. Infants gain infection through contaminated breast milk have a much higher recurrence rate compared to infants through other potential sources.
However, the importance of contaminated breast milk with GBS remains uncertain. GBS in breast milk is not likely to influence the healthy breastfed infants (9). The presence of maternal mastitis cannot be the criterion for breast milk contamination. The variety of delivery, treatment and storage methods of breast milk play a role in GBS contamination. There is no consensus on the time of the test of breast milk and whether breast-feeding should be ceased at the presence of GBS infection (2).
Another cause of GBS is horizontal transmission (healthcare workers, colonized parents, other environmental sources, like repeated medical equipment). The horizontal transmission of GBS is a preventable cause of morbidity and mortality of GBS disease. Additional research regarding the transmission of late-onset GBS infection and risk factors can help develop effective preventive methods and decrease the significant morbidity and mortality seen in this high-risk population.
Since 1996, antenatal GBS screening and intrapartum antimicrobial prophylaxis (IAP) has been recommended in US. The incidence of invasive EOD decreased greatly in US. Universal antenatal GBS screening and IAP for GBS-colonized women have been proved to be highly effective at preventing EOD, but they have no clear effect on LOD. The incidence of LOD was stable, approximately 0.26 per 1,000 live births (10). Since now, we have not identified an effective prevention of LOD.
However, there are inherent limitations to universal antenatal GBS screening and IAP. False negative prenatal screening results and the change of status during the screening time are inevitable. The time of screening, the collection of specimens and the disposal of cultures may account for the false negative results. Also, routine screening usually happens around 35 to 37 weeks’ gestation, so mothers delivering preterm may miss such opportunity. They partly account for higher rates of positive maternal results after the diagnosis of LOD. What’s more, it is impractical for many of the world’s poorest countries to implement countrywide intravenous IAP and antenatal GBS screening. A prospective cohort study proposed that IAP were associated with infant gut microbiota dysbiosis, but the long-term results of perturbation remain to be well-understood.
A GBS vaccine can be a greater promise for poorer countries. Four dominant serotypes (IA, IB, III and V) cause the 96.5% of LOD cases (10). These serotypes are also the major cause of EOD and adult GBS cases, so a vaccine against these serotypes is promising in many age groups. Recent studies suggest that maternal antibodies to type III, type IA and type V capsular polysaccharide (CPS) protect most neonates from CPS type III, type IA and type V GBS invasive disease in infants. GBS surface proteins are also candidate vaccine targets.
Here we reported a case of intracranial hemorrhage associated with GBS disease. The diagnosis was proved by the culture results and CT. Due to the poor condition, we did not have time to explore the true source of infection. The underlying mechanism remains unclear.
Intracranial hemorrhage caused by GBS is rare among the GBS disease. Also, the complications of infective endocarditis (IE) and cerebral infarction are rare in LOD. In clinic, doctors need to be aware of the complexity of bacterial infection. Also, it is required to have a better understanding of the transmission of LOD to prevent it. The role of polluted breast milk in the transmission of LOD deserves to be emphasized.
Acknowledgments
Funding: The present study was supported by grants from The Natural Science Foundation of Hunan Province, China (grant no. 2019JJ40461).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This case report was approved by the Ethics Committee of the Third Xiangya Hospital of Central South University (No. 2019-S529). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Russell NJ, Seale AC, O'Sullivan C, et al. Risk of early-onset neonatal group B streptococcal disease with maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S152-9. [Crossref] [PubMed]
- Zimmermann P, Gwee A, Curtis N. The controversial role of breast milk in GBS late-onset disease. J Infect 2017;74 Suppl 1:S34-40. [Crossref] [PubMed]
- Morgan K, Baca N. Late-onset group B streptococcal meningitis in infants. Pediatr Neurol 2015;53:175-6. [Crossref] [PubMed]
- Takahara T, Matsubara K, Maihara T, et al. Ultra-late-onset meningitis caused by serotype IX group B streptococcus. Pediatr Infect Dis J 2015;34:801. [Crossref] [PubMed]
- Hon KL, Chan KH, Ko PL, et al. Late onset streptococcus agalactiae meningitis following early onset septicemia: a preventable disease? Case Rep Pediatr 2017;2017:8418105. [Crossref] [PubMed]
- Bianchi-Jassir F, Seale AC, Kohli-Lynch M, et al. Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S133-42. [Crossref] [PubMed]
- Randis TM, Baker JA, Ratner AJ. Group B streptococcal infections. Pediatr Rev 2017;38:254-62. [Crossref] [PubMed]
- Cutland CL, Schrag SJ, Thigpen MC, et al. Increased risk for group B streptococcus sepsis in young infants exposed to HIV, Soweto, South Africa, 2004-2008(1). Emerg Infect Dis 2015;21:638-45. [Crossref] [PubMed]
- Le Doare K, Kampmann B. Breast milk and group B streptococcal infection: vector of transmission or vehicle for protection? Vaccine 2014;32:3128-32. [Crossref] [PubMed]
- Madrid L, Seale AC, Kohli-Lynch M, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S160-72. [Crossref] [PubMed]