Vitamin D deficiency in surgical congenital heart disease: prevalence and relevance
Population and problem
Congenital heart disease (CHD) is a common condition with an estimated prevalence of 1 per 100 in the general population (1). A significant proportion of these children require one or more corrective surgeries over their lifetime, collectively leading to 15,000 procedures per year in North America (1). Post-operatively, these patients may suffer significant morbidities which include a pronounced systemic inflammatory response, coagulopathy, respiratory failure, electrolyte disturbances, arrhythmia, myocardial dysfunction, kidney failure, infection and endocrine imbalances (2-5). Interventions that target one or multiple of these pathophysiological states could prevent illness, speed recovery, and decrease chronic morbidity in this high risk pediatric population.
Emerging literature suggests vitamin D deficiency to be a highly prevalent problem in the immediate post-operative CHD population. This observation is immediately relevant to researchers and clinicians since vitamin D is well recognized to be a pleiotropic hormone important for the proper functioning of organs critical to post-operative illness and outcome. Considered inexpensive and safe, vitamin D supplementation could prove to be an ideal intervention for improving outcomes in children with significant CHD.
Overview of vitamin D
Vitamin D axis
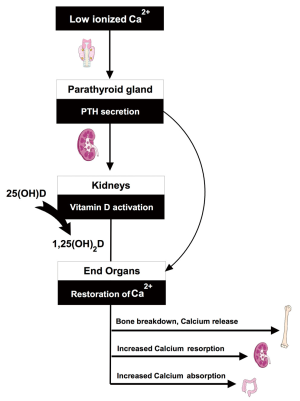
A schematic of the endocrine pathway is provided in Figure 1. The vitamin D axis is primarily understood in the context of total body and serum calcium homeostasis (6,7). In response to low ionized calcium, the parathyroid glands increase parathyroid hormone (PTH) secretion. Increased serum PTH leads to activation of vitamin D through an inducible renal enzyme, converting 25 hydroxyvitamin D (25OHD) to 1,25 dihydroxyvitamin D (1,25OH2D). The activated vitamin D, or calcitriol, works to restore serum calcium through bone breakdown, gastro-intestinal absorption, and increased renal reabsorption. Body stores of 25OHD are built and maintained through skin photosynthesis and dietary intake of pre-vitamin D that is immediately hydroxylated in the liver to 25OHD. Inadequate vitamin D intake through sun exposure and supplementation represent the most common reasons for diminished vitamin D axis functioning. Alternatively, or in addition, decreased renal or parathyroid function can result in low 1,25OH2D levels despite normal 25OHD levels (Figure 2).
Evaluation of vitamin D status
Circulating 25OHD is the accepted marker of vitamin D status, with three threshold ranges commonly cited in the literature. Generally, sufficiency is accepted as a value above 75-80 nmol/L, deficiency is defined as a value below 50 nmol/L, with severe deficiency occurring in the 25-30 nmol/L range (7-13). These thresholds are based on biochemical indicators of axis stress, and the values below which bone health or calcemic symptoms develop. Briefly, when 25OHD concentrations fall into the 50 nmol/L range, maintenance of active hormone levels requires elevation of serum parathyroid hormone (PTH) and increased renal enzyme activity (14,15). As 25OHD falls below the 25 to 30 nmol/L range, production of the active hormone (1,25OH2D) falls and otherwise healthy individuals can develop electrolyte disturbances and clinically evident disease (rickets, seizures, myocardial disease) (15-17). Although overt clinical disease is often not evident until values drop below 30 nmol/L, population based research has established improved bone health with values over 50 nmol/L (11). Further research confirming or refuting 50 nmol/L as the appropriate threshold for prevention of non-bone related disease is required.
The clinical and research assays available for 25OHD determination can be divided into two analytical approaches: Liquid Chromatography-Mass Spectrometry (LC-MS) and antibody based immunoassays (8,18). Although considered equivalent by some, there is emerging evidence that LC-MS may be superior for infants and young children. Superiority of the LC-MS methods relates to the ability to resolve 25OHD from a number of vitamin D metabolites that occur at relevant concentrations in infants and young children, particularly 24,25 dihydroxyvitamin D and 25-hydroxy-3-epi-vitamin D3 (3-epi-25OHD) (19-22). Recent studies have shown that the antibodies used in most immunoassay cross react with one or more of these metabolites, and counting these toward the 25OHD total may be inappropriate as they appear to have reduced or absent clinical effects (23).
Vitamin D deficiency in CHD patients
Previously described roles for vitamin D in the maintenance of electrolyte homeostasis, cardiovascular health, inflammation and innate immunity have lead multiple research groups to investigate and report on the prevalence of vitamin D deficiency among both critically ill children and post-operative CHD populations. Two studies on vitamin D in pediatric critical illness were published in 2012, one of which included a subgroup of 120 patients with CHD (24,25). This study reported that approximately seven out of every ten critically ill children were vitamin D deficient, and subgroup analysis on the post-operative CHD participants confirmed a 73% deficiency rate with a mean 25OHD level of 40 nmol/L (24). To identify potential center specific effects, post-operative 25OHD levels in CHD patients were compared at the three Canadian cardiac surgery sites and did not identify any statistically significant differences (26). A third PICU cohort study was published later in 2012 that reported a 40% vitamin D deficiency rate in post-operative cardiac surgery patients (27). In 2013 two studies have been published focusing specifically on the post-operative CHD population (26,28). Graham et al. used blood remaining from a glucocorticoid study of 70 neonates with CHD to show that 84% had 25OHD levels below 50 nmol/L (28).The other study prospectively evaluated 58 CHD patients with a range of ages and lesion types and found a mean post-operative value of 35 nmol/L and an 85% vitamin D deficiency rate (26). Findings summarized in Table 1.

Full table
Inspection of the 4 observational studies that included post-operative CHD patients demonstrates that all report statistically significant associations between lower vitamin D levels and need for cardiovascular support (vasopressor and/or inotropes). Findings from the sole PICU study that did not include post-operative cardiac patients also demonstrated a link between lower vitamin D levels and cardiovascular function (25). In addition, four studies showed an association between vitamin D levels and at least one other clinically important outcome measure including Pediatric Risk of Mortality III scores (25,29), hypocalcemia (24) or calcium supplementation (27), fluid requirements (24), and PICU length of stay (24). Findings are summarized in Table 1; illness severity associations shown for the McNally et al. cohort study represent those for the entire group, while those for the study by Rippel and colleagues represent the CHD subgroup.
Additional evidence supporting vitamin D deficiency as a modifiable risk factor for poor outcome following CHD surgery can be found in a growing number of observational studies in adult ICU populations (30-38). A detailed evaluation of the adult critical care literature is beyond the scope of this article and has been reviewed elsewhere (39,40). Briefly, the first adult publication on the topic in 2009 described 42 ICU patients, reported an average 25OHD level of 40 nmol/L, and demonstrated greater illness severity scores with lower hormone levels (30). Following a number of supportive small observational studies Braun et al. confirmed the association between lower admission vitamin D and mortality in a large observational study involving thousands of adult patients (41). Separately, this same research group was also able to demonstrate pre-illness 25OHD concentration as a predictor of subsequent ICU related mortality (33). Although vitamin D deficiency has been associated with cardiovascular disease (36,37,42), the potential relevance of vitamin D deficiency to outcomes following adult cardiac surgery remains less well defined (43,44). The best study to date comes from large prospective observational study published this year by Zitterman and colleagues, wherein they demonstrated that compared to normal 25OHD levels (75-100 nmol/L) having a value below 50 nmol/L was significantly associated with MACCE risk (in-hospital death, MI, length of stay or stroke) (44).
Factors contributing to post-operative vitamin D deficiency
There are multiple pre-operative, intraoperative and immediate post-operative factors that may contribute to post-operative vitamin D deficiency.
Primary vitamin D deficiency
Children with CHD may be at increased risk for pre and post-operative vitamin D deficiency due to inadequate sun exposure and poor vitamin D intake, that may be related to their underlying disease. Significant pre-surgical vitamin D deficiency rates were described in both pediatric studies that measured 25OHD levels pre-operatively (26,28). This observation suggests either poor compliance with guidelines or that vitamin D requirements (metabolism) differ for CHD patients compared with healthy children. The question of compliance with current recommendations (9,11,12) for vitamin D intake and supplementation was addressed through a targeted vitamin D food frequency questionnaire in the prospective study performed by McNally et al. (26). This questionnaire demonstrated that up to 50% were not achieving a daily vitamin D intake of 400 IU. This non-compliance with vitamin D recommendations occurred despite close supervision by a large group of health care providers and has also been reported in the general population (45,46).
Secondary vitamin D deficiency
In addition to the mechanisms outlined above, blood vitamin D levels may be further reduced either intra-operatively or immediate post-operatively due to large circulating fluid shifts, blood loss, blood ultrafiltration, fluid administration and interstitial leak of vitamin D binding proteins due to inflammation (47-50). This occurrence, and timing, was explored by McNally et al. through the collection of serial blood samples through surgery and over the first two post-operative days (26). The major finding was a 40% intraoperative fall in serum 25OHD immediately following initiation of CPB. Similar blood levels before and after modified ultrafiltration (MUF) and insignificant 25OHD in the ultra-filtrate do not support a loss of vitamin D through ultrafiltation. Other possibilities include either a dilutional effect from the prime volume or absorption of 25OHD by components of the bypass circuit (e.g., tubing, oxygenator membrane). No further change in group mean 25OHD levels occurred after PICU admission, evaluated over the critical first two post-operative days.
It is worth noting that in the retrospective study by Graham and colleagues an intra-operative decline in 25OHD concentration was not observed (28). The lack of even a small decline is not consistent with the study by McNally et al., two recent adult CPB studies and a small neonatal case series describing calcitriol concentrations before and after initiation of ECMO (26,47,51,52). Zitterman et al. reported a significant drop in 25OHD level following adult heart transplantation, but the exact timing could not be determined as the first post-operative levels were measured on the sixth post-operative day (51). The study by Krishnan and colleagues, demonstrated up to a 40% drop in serum 25OHD in 19 adults following the CPB prime (47). A potential explanation for the contrast in findings between Graham et al. and the other four studies could be a result of differences in research blood collection, processing and storage. Blood collection approaches may be essential as 25OHD levels have been shown to be consistently 20% higher when measured from capillary blood compared to venous blood (53). Further, and as noted by Graham and colleagues, the pre-operative vitamin D level and choice of CPB prime fluids may also contribute to the differences in findings (28).
Possible mechanisms of vitamin D deficiency in post-cardiac surgery patients
The role of vitamin D as a modifiable risk factor for post-cardiac surgery outcome has biological plausibility due to the number of known organs and body pathways through which it could cause or worsen post-operative pathophysiology.
Critical illness hypocalcemia
Hypocalcemia is a common problem following pediatric cardiac surgery (~30%), with a 2008 study demonstrating the need for calcium supplementation as a risk factor for morbidity and mortality (5). Calcium homeostasis is important for patient well-being as calcium initiates and propagates nerve conduction, muscle contraction, and contributes to intra-cellular signal transduction. In addition to the negative impacts of hypocalcemia on cardiovascular dysfunction, abnormal calcium homeostasis could impair gas exchange and influence ventilator requirements through nerve dysfunction and muscle weakness (54,55). A number of pediatric studies have confirmed hypocalcemia to be a risk factor for worse ICU outcomes and that critically ill children with hypocalcemia are more likely to have abnormalities of their vitamin D axis, including 25OHD deficiency (56-58). No studies have evaluated whether optimization of vitamin D status prevents or reduces the severity of critical illness hypocalcemia.
Cardiovascular dysfunction
Post-operative cardiovascular dysfunction is common following cardiac surgery with many children requiring the continuous infusion of one or more vasoactive medications to support blood pressure and maintain cardiac output (59). A role for vitamin D in pediatric cardiac health can be found in case reports and case series describing cardiomyopathy secondary to isolated severe vitamin D deficiency (60-65). A recent case series identified 16 children with treatment responsive cardiomyopathy secondary to isolated severe vitamin D deficiency (63). Vitamin D responsive subclinical cardiac dysfunction has also been described in childhood rickets, with 50% of one cohort demonstrating ECG and echocardiogram abnormalities at presentation (66). In addition to the indirect actions of vitamin D through calcium, it is well known that vitamin D influences myocyte structure and function via nuclear vitamin D receptors that alter gene and protein expression (67,68). More recent research has also suggested that vitamin D may mediate structural and functional myocyte changes through non-nuclear VDRs (29). As an example, myocyte contractility has been observed to be favorably altered within minutes following 1,25OH2D supplementation, an effect mediated through signal transduction pathways, enzymatic reactions and ion channels (69,70). Further, vitamin D appears to play a role modulating peripheral vascular resistance directly through receptors on smooth muscle cells and indirectly through the renin-angiotensin-aldosterone system (32). Finally numerous studies have recently suggested that vitamin D deficiency could serve as an effect modifier, augmenting the impact of other deficiency states (e.g., adrenal) and medications (corticosteroids) on cardiorespiratory function (71-73).
Immune dysfunction
Cardiac surgery uniformly leads to a post-operative systemic inflammatory response that can contribute significantly to low cardiac output and respiratory dysfunction (74,75). There is good evidence that vitamin D metabolites play important immunomodulatory roles mediated through functional vitamin D receptors present on all major immune cell types. Specifically, vitamin D has been demonstrated to inhibit antigen-induced T-cell proliferation, antagonize the pro-inflammatory Th1 (T-helper) response, suppress macrophage release of pro-inflammatory cytokines, and alter gene expression of adhesion factors, decreasing adherence and chemotaxis of neutrophils (76-78). Vitamin D signaling is also known to play a role in innate immunity through the production of cathelicidins which are important endogenous antimicrobial peptides that provide protection again multiple viral and bacterial pathogens (79-81).
Vitamin D supplementation regimens
The cumulative body of basic science and clinical literature suggests that optimization of vitamin D status could improve clinical outcomes in CHD patients. Available evidence suggests that both primary deficiency and operative procedures potentially contribute to high post-operative deficiency rates. Although rapid restoration of vitamin D levels immediately following surgery would represent an attractive option for anesthesiology and intensivists, the lack an intravenous formulation of cholecalciferol or 25-hydroxyvitamin D prevents consideration of such an approach. Instead, the practitioner caring for children with CHD will need to pre-operatively raise and maintain levels utilizing one of two approaches proven for other pediatric populations. Given the pharmacokinetics of vitamin D coma prevention of post-operative deficiency patients may necessitate an understanding of both approaches, with application personalized based on patients factors such as compliance with daily intake and time prior to surgery.
Considering the primary literature, expert opinion and concerns about post-surgical inflammation, hypoparathyroidism and renal dysfunction, it would be ideal to target post-operative 25OHD level above 75 nmol/L, with the goal of avoiding values below 50 nmol/L. Given the potential for a significant (40%) intra-operative decline, pre-operative levels in the 100-150 nmol/L range may be required to achieve these goals.
Daily low dose vitamin D supplementation
The most commonly used approach for building and maintaining levels of vitamin D relies on the daily consumption of an age specific low dose of ergocalciferol or cholecalciferol (ranging from 400 to 4,000 IU). To date there have been no pediatric trials of any vitamin D regimen in the CHD population, necessitating extrapolation of recommendations from the most recent guidelines or position statements for healthy children (9,11,12). Recently, at the request of agencies of the US and Canadian governments, the Institute of Medicine (IOM) assembled a committee to provide recommendation for intake of vitamin D based upon a rigorous and comprehensive review of literature. In the final report, the IOM provided two age specific doses: (I) Recommended Daily Allowance or Adequate Intake and (II) Tolerable Upper Intake Level (UL) (11). As described in the IOM report, the Recommended Daily Allowance and Adequate Intake doses are intended to maintain blood 25OHD concentrations at or slightly above the 25OHD threshold (50 nmol/L) known to foster bone health. In calculating the age specific upper intake level, the IOM goal was to provide a daily dose that would significant elevate levels well above the cut-off for vitamin D deficiency while safely avoiding potential toxicity.
The efficacy and safety of the two age specific IOM dosing regimens have been supported by the publication of two well done trials since the IOM report (19,82). First, a Canadian study confirmed previous work demonstrating that 2 or more months of daily dosing is required to achieve a new steady state 25OHD level (19). Second, both studies showed that with good compliance, 400 IU per day for 3 months will generate a mean pre-operative level of 80-90 nmol/L, with almost all participants elevated above 50 nmol/L. However, these two studies also suggest that utilization of this dose in CHD patients could leave 50% or more at risk for post-operative vitamin D deficiency. Both studies evaluated a 1,600 IU/day regimen, closely approximating the 6 to 12 month old upper intake level. At this dose, the mean 25OHD concentrations achieved in the two studies were 157 and 180 nmol/L, with almost all generating 3 month levels above 90 nmol/L. Importantly, after consideration of unwanted metabolites, no child exceeded a 25OHD level above 250 nmol/L. Neither study demonstrated additional cases of hypercalcemia or hypercalciuria in the groups receiving doses above standard of care.
Intermittent high dose supplementation
Some circumstances may necessitate consideration of an alternative approach to the daily low dose vitamin D regimen. First, poor compliance with vitamin D supplementation occurs in a certain percentage of patients as previously demonstrated in the general population (45,46). Second, in a small but consistent percentage of the CHD population, particularly neonates and young infants, the time between diagnosis and surgery will not allow for 2 to 3 months of low dose vitamin D intake.
The second approach to supplementation involves the delivery of a 2 to 3 month prescription of vitamin D either as a single dose or over a period of days (83-85). This regimen is commonly referred to as Stosstherapy (or megadose therapy) and generally involves the oral or intramuscular adminitration of 50,000 to 600,000. Not surprisingly the route of administration contributes significantly to the rate of change and final 25OHD level achieved. When given orally, the loading dose is immediately absorbed into the circulation, undergoes rapid liver hydroxylation, and gives rise to a peak 25OHD within a few days (17,86-88). In contrast, with intramusclar administration, the rise in 25OHD levels occurs over many weeks due to slow resorption from the muscle, and may be more variable (89).
There is significant experience with the high dose regimen in certain regions of the world and it has been recommended as part of the Australia and New Zealand position statement (90). However, it is important to point out that the available pediatric clinical trials have largely focused on healthy children (without or without vitamin D deficiency) and administered doses intended to prevent or treat vitamin D related bone disease. The safety of this dose regimen has received limited evaluation in children with cardiac dysfunction or acute illness particular at doses intended to achieve 25OHD levels in the 100 to 150 nmol/L range. As there is evidence to suggest that some populations at certain doses may develop hypercalcemia additional work in this area will be required to determine the high dose supplemention approach that safely maximizes 25OHD level in CHD patients (91).
Vitamin D toxicity
Signs and symptoms
Vitamin D toxicity is a characterized by hypercalcemia and/or hypercalciuria, with the classic symptoms (lethargy, abdominal pain, anorexia, constipation, polyuria and nocturia) directly attributable to these abnormalities. In many instances symptomatology related to hypercalcemia and/or hypercalciuria is minor. However, as documented in case reports and case series the longstanding persistence of minor biochemical abnormalities or progression to severe electrolyte disturbances can give rise to more serious problems including dehydration, renal dysfunction and eventual nephrocalcinosis.
Toxic threshold levels
Presently no 25OHD level has been universally accepted as the threshold above which risk develops, with authors generally citing values between 250 and 750 nmol/L. Although there is no evidence that children develop biochemical abnormalities or symptoms with 25OHD values at or slightly above 250 nmol/L, recent pediatric clinical trials of high dose vitamin D have focused on this threshold (19,82). Application of this threshold for dosing studies is appropriate given that levels above this are supraphyiosological (cannot be achieved with excessive sun-exposure or healthy diets) and there is no evidence of benefit for 25OHD doses above 200 nmol/L (92,93).
Risk factors for vitamin D related toxicity
Despite public and clinician concern regarding vitamin D toxicity, it is a rare event that generally occurs in the context of genetic susceptibility or inappropriate intake of high doses of vitamin D.
Concern about the safety of daily vitamin D supplementation above 400 IU/day dates back to the 1950’s when a rise in idiopathic infantile hypercalcemia (IIH) cases coincided with the population based implementation of increased daily vitamin D intakes to ~4,000 IU/day (94-98). This small epidemic led to a decrease in recommended daily intake to levels known to prevent rickets and hypocalcemic seizures (400 IU/day). It has recently been argued that many, perhaps all, cases of IIH were due to rare genetic conditions (<1:10,000) that increase susceptibility to vitamin D toxicity (99). Of these, patients with Williams syndrome can have heart defects as part of their constellation of symptoms and it would be prudent to avoid higher vitamin D intake in this subgroup (100).
There is a substantial body of low level evidence suggesting that high dose vitamin D giving rise to shorter term cumulative intake at or above 600,000 IU is excessive and can lead to hypercalcemia, hypercalciuria and eventual nephrocalcinosis. This anecdotal evidence is also supported by one prospective pediatric clinical trial that demonstrated significant hypercalcemia rates among healthy infants who received intermittent, often repeated, high dose therapy with 600,000 IU (91). In most instances diagnosis and discontinuation of the vitamin D source results in a gradual decline of blood levels below toxic levels and resolution of symptoms and biochemical abnormalities. In some circumstances the nephrocalcinosis persisted despite discontinuation of vitamin D. A review of the literature on nephrocalcinosis shows that most cases associated with vitamin D have occurred in children with a rare genetic disorder called vitamin D resistant rickets and may be related to concurrent phosphate intake (101-105). Again, a review of case series and case reports demonstrate that otherwise healthy children only develop nephrocalcinosis following intentional or unintentional cumulative vitamin D intake above 600,000 (101,102,106,107). Our review of pediatric interventional trials on vitamin D supplementation identified 2 studies evaluating daily high dose vitamin D approximating below the IOM upper intake level and 4 with megadoses (100,000 to 150,000 IU range); none identified increased urinary calcium excretion or hypercalciuria (19,82,108-111). Given these findings it would be prudent to avoid oral vitamin D dosing at or near 600,000 IU.
Future directions
Absence of clinical trial evidence on ergocalciferol or cholecalciferol
As stated previously, there are no studies evaluating ergocalciferol or cholecalciferol dosing in CHD patients. Although sufficient evidence exists to support administration of vitamin D regimens 2 to 4 times above the current standard of care (400-600 IU/day), extrapolation of these safety findings from healthy infants to the CHD population may not be appropriate. CHD patients have unique metabolic demands, organ dysfunctions, and known and unknown genetic abnormalities that may make them more or less susceptible to vitamin D toxicity (100,112-114). Similar to the recent approach taken from other severely ill populations, including pediatric heart failure (115), pediatric acute lower respiratory tract infection (116,117) and adult critical illness (88,118) it would be prudent to test feasible dosing regimens as part of phase II clinical trials.
Supplementation with active vitamin D hormone
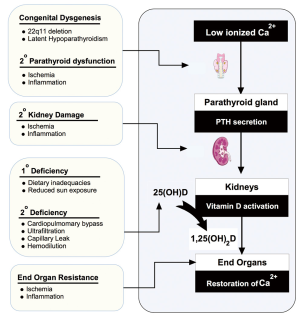
Although recognized as the best indicator of vitamin D status, post-operative 25OHD concentration may not accurately reflect vitamin D axis functioning (and calcitriol levels) in the immediate post-operative CHD patient. As shown in Figure 3, the post-operative CHD patient has many congenital, pre-operative and surgically acquired risk factors for low post-operative calcitriol for reasons beyond impaired 25OHD levels. Of these, we speculate that CPB may lead to a significant acute decline in blood calcitriol levels, as demonstrated in studies following adult cardiac transplant and initiation of neonatal ECMO (51,52). Further, as described in other patient populations, pre-surgical or acquired dysfunction of the parathyroid and renal organs may limit or prevent conversion of 25OHD to calcitriol (7,119). If future studies report a transient or persistent decline in calcitriol levels following CPB, the administration of active vitamin D may be required to achieve the goal of optimizing vitamin D status. Clinical evidence demonstrating cardiovascular benefit of calcitriol administration, in addition to 25OH, can be found in the end stage renal disease literature, where a vitamin D deficient state emerges due to reduced renal activation of vitamin D. In this population, the administration of an activated vitamin D analogue reduces the 5 to 10 fold increase in cardiovascular disease (18,120).
Nutrition and other vitamins
The impact of cardiac surgery and CPB on the other endocrine axis regulating cortisol, glucose, thyroid and vasopressin have been well described. However, the impact of cardiac surgery and CPB on other vitamins and blood metabolites remains less well defined. A better understanding of how these metabolites respond to CPB and post-surgical care would inform researchers regarding the need for multivitamin supplementation in patients undergoing CPB.
Conclusions
Multiple observational studies have identified significant rates of post cardiac surgery vitamin D deficiency in a high risk (CHD) pediatric population following CPB. Data from these same studies demonstrate that lower post-operative hormone levels are associated with a more protracted clinical course. Available data strongly suggests that the current approach to vitamin D supplementation will not reliably prevent post-operative vitamin D deficiency. The current lack of clinical trials evaluating the efficacy or safety of any alternative high dose vitamin D regimen prevents evidence based recommendation. At a minimum clinicians looking after patients with CHD are encouraged to promote compliance with current RDA and may consider the higher daily doses of vitamin D recommended by the IOM if close follow-up is planned. Systematic reviews of the available literature combined with clinical trials will be required to explore alternative vitamin D supplementation strategies that will safely and effectively optimize post-operative vitamin D status in most CHD patients.
Acknowledgements
The authors thank Loren Matheson for proof reading and editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The authors disclose that a similar review has not been submitted concurrently to another journal.
References
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [PubMed]
- Brix-Christensen V. The systemic inflammatory response after cardiac surgery with cardiopulmonary bypass in children. Acta Anaesthesiol Scand 2001;45:671-9. [PubMed]
- Gazit AZ, Huddleston CB, Checchia PA, et al. Care of the pediatric cardiac surgery patient--part 1. Curr Probl Surg 2010;47:185-250. [PubMed]
- McEwan A. Aspects of bleeding after cardiac surgery in children. Paediatr Anaesth 2007;17:1126-33. [PubMed]
- Dyke PC 2nd, Yates AR, Cua CL, et al. Increased calcium supplementation is associated with morbidity and mortality in the infant postoperative cardiac patient. Pediatr Crit Care Med 2007;8:254-7. [PubMed]
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008;359:391-403. [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [PubMed]
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 2009;19:73-8. [PubMed]
- Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatr Child Health 2007;12:583-98. [PubMed]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [PubMed]
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53-8. [PubMed]
- Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142-52. [PubMed]
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc 2011;86:50-60. [PubMed]
- Willett AM. Vitamin D status and its relationship with parathyroid hormone and bone mineral status in older adolescents. Proc Nutr Soc 2005;64:193-203. [PubMed]
- Christensen MH, Lien EA, Hustad S, et al. Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest 2010;70:281-6. [PubMed]
- Docio S, Riancho JA, Pérez A, et al. Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 1998;13:544-8. [PubMed]
- Thacher TD, Fischer PR, Isichei CO, et al. Early response to vitamin D2 in children with calcium deficiency rickets. J Pediatr 2006;149:840-4. [PubMed]
- Hart GR, Furniss JL, Laurie D, et al. Measurement of vitamin D status: background, clinical use, and methodologies. Clin Lab 2006;52:335-43. [PubMed]
- Gallo S, Comeau K, Vanstone C, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309:1785-92. [PubMed]
- van den Ouweland JM, Vogeser M, Bächer S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev Endocr Metab Disord 2013;14:159-84. [PubMed]
- Wright MJ, Halsall DJ, Keevil BG. Removal of 3-epi-25-hydroxyvitamin D(3) interference by liquid chromatography-tandem mass spectrometry is not required for the measurement of 25-hydroxyvitamin D(3) in patients older than 2 years. Clin Chem 2012;58:1719-20. [PubMed]
- Singh RJ, Taylor RL, Reddy GS, et al. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 2006;91:3055-61. [PubMed]
- Bailey D, Veljkovic K, Yazdanpanah M, et al. Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin Biochem 2013;46:190-6. [PubMed]
- McNally JD, Menon K, Chakraborty P, et al. The association of vitamin D status with pediatric critical illness. Pediatrics 2012;130:429-36. [PubMed]
- Madden K, Feldman HA, Smith EM, et al. Vitamin D deficiency in critically ill children. Pediatrics 2012;130:421-8. [PubMed]
- McNally JD, Menon K, Chakraborty P, et al. Impact of anesthesia and surgery for congenital heart disease on the vitamin D status of infants and children: a prospective longitudinal study. Anesthesiology 2013;119:71-80. [PubMed]
- Rippel C, South M, Butt WW, et al. Vitamin D status in critically ill children. Intensive Care Med 2012;38:2055-62. [PubMed]
- Graham EM, Taylor SN, Zyblewski SC, et al. Vitamin D status in neonates undergoing cardiac operations: relationship to cardiopulmonary bypass and association with outcomes. J Pediatr 2013;162:823-6. [PubMed]
- van Keulen JG, Polderman KH, Gemke RJ. Reliability of PRISM and PIM scores in paediatric intensive care. Arch Dis Child 2005;90:211-4. [PubMed]
- Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med 2009;360:1912-4. [PubMed]
- Lucidarme O, Messai E, Mazzoni T, et al. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med 2010;36:1609-11. [PubMed]
- McKinney JD, Bailey BA, Garrett LH, et al. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc 2011;12:208-11. [PubMed]
- Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med 2011;39:671-7. [PubMed]
- Matthews LR, Ahmed Y, Wilson KL, et al. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg 2012;204:37-43. [PubMed]
- Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340-9. [PubMed]
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008;93:3927-35. [PubMed]
- Giovannucci E, Liu Y, Hollis BW, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008;168:1174-80. [PubMed]
- Higgins DM, Wischmeyer PE, Queensland KM, et al. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN J Parenter Enteral Nutr 2012;36:713-20. [PubMed]
- Sauneuf B, Brunet J, Lucidarme O, et al. Prevalence and risk factors of vitamin D deficiency in critically ill patients. Inflamm Allergy Drug Targets 2013;12:223-9. [PubMed]
- Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab 2011;25:769-81. [PubMed]
- Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med 2012;40:63-72. [PubMed]
- Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol 2003;41:105-12. [PubMed]
- Turan A, Grady M, You J, et al. Low vitamin d concentration is not associated with increased mortality and morbidity after cardiac surgery. PLoS One 2013;8:e63831. [PubMed]
- Zittermann A, Kuhn J, Dreier J, et al. Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur Heart J 2013;34:1358-64. [PubMed]
- Perrine CG, Sharma AJ, Jefferds ME, et al. Adherence to vitamin D recommendations among US infants. Pediatrics 2010;125:627-32. [PubMed]
- Gallo S, Jean-Philippe S, Rodd C, et al. Vitamin D supplementation of Canadian infants: practices of Montreal mothers. Appl Physiol Nutr Metab 2010;35:303-9. [PubMed]
- Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care 2010;14:R216. [PubMed]
- Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr 2011;93:1006-11. [PubMed]
- Meier U, Gressner O, Lammert F, et al. Gc-globulin: roles in response to injury. Clin Chem 2006;52:1247-53. [PubMed]
- Speeckaert MM, Wehlou C, De Somer F, et al. Evolution of vitamin D binding protein concentration in sera from cardiac surgery patients is determined by triglyceridemia. Clin Chem Lab Med 2010;48:1345-50. [PubMed]
- Zittermann A, Schleithoff SS, Götting C, et al. Calcitriol deficiency and 1-year mortality in cardiac transplant recipients. Transplantation 2009;87:118-24. [PubMed]
- Hak EB, Crill CM, Bugnitz MC, et al. Increased parathyroid hormone and decreased calcitriol during neonatal extracorporeal membrane oxygenation. Intensive Care Med 2005;31:264-70. [PubMed]
- Dayre McNally J, Matheson LA, Sankaran K, et al. Capillary blood sampling as an alternative to venipuncture in the assessment of serum 25 hydroxyvitamin D levels. J Steroid Biochem Mol Biol 2008;112:164-8. [PubMed]
- Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet 1976;1:626-9. [PubMed]
- Skaria J, Katiyar BC, Srivastava TP, et al. Myopathy and neuropathy associated with osteomalacia. Acta Neurol Scand 1975;51:37-58. [PubMed]
- Gauthier B, Trachtman H, Di Carmine F, et al. Hypocalcemia and hypercalcitoninemia in critically ill children. Crit Care Med 1990;18:1215-9. [PubMed]
- Broner CW, Stidham GL, Westenkirchner DF, et al. Hypermagnesemia and hypocalcemia as predictors of high mortality in critically ill pediatric patients. Crit Care Med 1990;18:921-8. [PubMed]
- Cardenas-Rivero N, Chernow B, Stoiko MA, et al. Hypocalcemia in critically ill children. J Pediatr 1989;114:946-51. [PubMed]
- Menon K, Ward RE, Lawson ML, et al. A prospective multicenter study of adrenal function in critically ill children. Am J Respir Crit Care Med 2010;182:246-51. [PubMed]
- Olgun H, Ceviz N, Ozkan B. A case of dilated cardiomyopathy due to nutritional vitamin D deficiency rickets. Turk J Pediatr 2003;45:152-4. [PubMed]
- Price DI, Stanford LC Jr, Braden DS, et al. Hypocalcemic rickets: an unusual cause of dilated cardiomyopathy. Pediatr Cardiol 2003;24:510-2. [PubMed]
- Verma S, Khadwal A, Chopra K, et al. Hypocalcemia nutritional rickets: a curable cause of dilated cardiomyopathy. J Trop Pediatr 2011;57:126-8. [PubMed]
- Maiya S, Sullivan I, Allgrove J, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart 2008;94:581-4. [PubMed]
- Brunvand L, Haga P, Tangsrud SE, et al. Congestive heart failure caused by vitamin D deficiency? Acta Paediatr 1995;84:106-8. [PubMed]
- Kosecik M, Ertas T. Dilated cardiomyopathy due to nutritional vitamin D deficiency rickets. Pediatr Int 2007;49:397-9. [PubMed]
- Uysal S, Kalayci AG, Baysal K. Cardiac functions in children with vitamin D deficiency rickets. Pediatr Cardiol 1999;20:283-6. [PubMed]
- Tishkoff DX, Nibbelink KA, Holmberg KH, et al. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008;149:558-64. [PubMed]
- Nibbelink KA, Tishkoff DX, Hershey SD, et al. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007;103:533-7. [PubMed]
- Santillán GE, Vazquez G, Boland RL. Activation of a beta-adrenergic-sensitive signal transduction pathway by the secosteroid hormone 1,25-(OH)2-vitamin D3 in chick heart. J Mol Cell Cardiol 1999;31:1095-104. [PubMed]
- Green JJ, Robinson DA, Wilson GE, et al. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006;41:350-9. [PubMed]
- McNally JD, Doherty DR, Lawson ML, et al. The relationship between vitamin D status and adrenal insufficiency in critically Ill children. J Clin Endocrinol Metab 2013;98:E877-81. [PubMed]
- Ahmed MA. Impact of vitamin D3 on cardiovascular responses to glucocorticoid excess. J Physiol Biochem 2013;69:267-76. [PubMed]
- Gupta A, Sjoukes A, Richards D, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med 2011;184:1342-9. [PubMed]
- Jaggers J, Lawson JH. Coagulopathy and inflammation in neonatal heart surgery: mechanisms and strategies. Ann Thorac Surg 2006;81:S2360-6. [PubMed]
- Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg 2006;81:S2347-54. [PubMed]
- Baeke F, Gysemans C, Korf H, et al. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol 2010;25:1597-606. [PubMed]
- Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest 1987;79:1659-64. [PubMed]
- Bhalla AK, Amento EP, Serog B, et al. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 1984;133:1748-54. [PubMed]
- Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol 2008;122:829-31. [PubMed]
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005;19:1067-77. [PubMed]
- Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 2009;7:28. [PubMed]
- Holmlund-Suila E, Viljakainen H, Hytinantti T, et al. High-dose vitamin d intervention in infants--effects on vitamin d status, calcium homeostasis, and bone strength. J Clin Endocrinol Metab 2012;97:4139-47. [PubMed]
- Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr 1994;125:487-90. [PubMed]
- Emel T, Doğan DA, Erdem G, et al. Therapy strategies in vitamin D deficiency with or without rickets: efficiency of low-dose stoss therapy. J Pediatr Endocrinol Metab 2012;25:107-10. [PubMed]
- Soliman AT, El-Dabbagh M, Adel A, et al. Clinical responses to a mega-dose of vitamin D3 in infants and toddlers with vitamin D deficiency rickets. J Trop Pediatr 2010;56:19-26. [PubMed]
- Argao EA, Heubi JE. Fat-soluble vitamin deficiency in infants and children. Curr Opin Pediatr 1993;5:562-6. [PubMed]
- Cipriani C, Romagnoli E, Scillitani A, et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab 2010;95:4771-7. [PubMed]
- Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care 2011;15:R104. [PubMed]
- Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab 2008;93:3015-20. [PubMed]
- Munns C, Zacharin MR, Rodda CP, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 2006;185:268-72. [PubMed]
- Markestad T, Hesse V, Siebenhuner M, et al. Intermittent high-dose vitamin D prophylaxis during infancy: effect on vitamin D metabolites, calcium, and phosphorus. Am J Clin Nutr 1987;46:652-8. [PubMed]
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999;69:842-56. [PubMed]
- Hollis BW, Wagner CL, Drezner MK, et al. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol 2007;103:631-4. [PubMed]
- Rhaney K, Mitchell RG. Idiopathic hypercalcaemia of infants. Lancet 1956;270:1028-32. [PubMed]
- Mitchell RG. The prognosis in idiopathic hypercalcaemia of infants. Arch Dis Child 1960;35:383-8. [PubMed]
- Lightwood R, Stapleton T. Idiopathic hypercalcaemia in infants. Lancet 1953;265:255-6. [PubMed]
- Bongiovanni A, Eberlein WR, Jones IT. Idiopathic hypercalcemia of infancy, with failure to thrive; report of three cases, with a consideration of the possible etiology. N Engl J Med 1957;257:951-8. [PubMed]
- Rhaney K, Mitchell RG. Idiopathic hypercalcaemia of infants. Lancet 1956;270:1028-32. [PubMed]
- Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 2011;365:410-21. [PubMed]
- Committee on Genetics. American Academy of Pediatrics: Health care supervision for children with Williams syndrome. Pediatrics 2001;107:1192-204. [PubMed]
- Ammenti A, Pelizzoni A, Cecconi M, et al. Nephrocalcinosis in children: a retrospective multi-centre study. Acta Paediatr 2009;98:1628-31. [PubMed]
- Rönnefarth G, Misselwitz J. Nephrocalcinosis in children: a retrospective survey. Members of the Arbeitsgemeinschaft für pädiatrische Nephrologie. Pediatr Nephrol 2000;14:1016-21. [PubMed]
- Moncrieff MW, Chance GW. Nephrotoxic effect of vitamin D therapy in vitamin D refractory rickets. Arch Dis Child 1969;44:571-9. [PubMed]
- Mantan M, Bagga A, Virdi VS, et al. Etiology of nephrocalcinosis in northern Indian children. Pediatr Nephrol 2007;22:829-33. [PubMed]
- Alon US. Nephrocalcinosis. Curr Opin Pediatr 1997;9:160-5. [PubMed]
- Atabek ME, Pirgon O, Sert A. Oral alendronate therapy for severe vitamin D intoxication of the infant with nephrocalcinosis. J Pediatr Endocrinol Metab 2006;19:169-72. [PubMed]
- Chambellan-Tison C, Horen B, Plat-Wilson G, et al. Severe hypercalcemia due to vitamin D intoxication. Arch Pediatr 2007;14:1328-32. [PubMed]
- Oliveri B, Cassinelli H, Mautalen C, et al. Vitamin D prophylaxis in children with a single dose of 150000 IU of vitamin D. Eur J Clin Nutr 1996;50:807-10. [PubMed]
- Duhamel JF, Zeghoud F, Sempé M, et al. Prevention of vitamin D deficiency in adolescents and pre-adolescents. An interventional multicenter study on the biological effect of repeated doses of 100,000 IU of vitamin D3. Arch Pediatr 2000;7:148-53. [PubMed]
- Mallet E, Philippe F, Castanet M, et al. Administration of a single Winter oral dose of 200,000 IU of vitamin D3 in adolescents in Normandy: evaluation of the safety and vitamin D status obtained. Arch Pediatr 2010;17:1042-6. [PubMed]
- Emel T, Doğan DA, Erdem G, et al. Therapy strategies in vitamin D deficiency with or without rickets: efficiency of low-dose stoss therapy. J Pediatr Endocrinol Metab 2012;25:107-10. [PubMed]
- Pierpont ME, Basson CT, Benson DW Jr, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007;115:3015-38. [PubMed]
- Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet 2007;370:1443-52. [PubMed]
- Zeigler VL. Congenital heart disease and genetics. Crit Care Nurs Clin North Am 2008;20:159-69. v. [PubMed]
- Shedeed SA. Vitamin D supplementation in infants with chronic congestive heart failure. Pediatr Cardiol 2012;33:713-9. [PubMed]
- Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 2010;15:1148-55. [PubMed]
- Choudhary N, Gupta P. Vitamin D supplementation for severe pneumonia--a randomized controlled trial. Indian Pediatr 2012;49:449-54. [PubMed]
- Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, et al. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol 2010;121:452-5. [PubMed]
- Zittermann A, Schulz U, Lazouski K, et al. Association between glomerular filtration rate and 1,25-dihydroxyvitamin D in cardiac surgery. Scand Cardiovasc J 2012;46:359-65. [PubMed]
- Yazdanpanah M, Bailey D, Walsh W, et al. Analytical measurement of serum 25-OH-vitamin D(3), 25-OH-vitamin D(2) and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin Biochem 2013;46:1264-71. [PubMed]


