Three-dimensional modelling and three-dimensional printing in pediatric and congenital cardiac surgery
‘The heart is sufficiently complicated to easily accommodate to all our ideas’—Robert H. Anderson.
Introduction
Advances in anatomy paved the way for modern medicine by abstracting features of individual anatomic representations into general rules (1). Anatomical demonstrations were mostly helped by three-dimensional (3D) ex vivo specimens as well as two-dimensional (2D) diagrams (2). Nowadays, 3D imaging methods greatly support the development of individualized medicine and surgery. In the cardiovascular domain, these imaging technologies include 3D echocardiography/ultrasound, 3D rotational angiography, computer-tomography (CT) and magnetic resonance (MR) imaging. They provide accurate direct information of the anatomy and in/directly of the hemodynamic consequences (3). Image data integration of these modalities will enhance 3D multimodality modelling with grossly improved reliability, accuracy and resolution (4). However, as images are currently not acquired in real time, three limitations persist: (I) any change in the position of patient or equipment can cause misalignment of the registration; (II) static reconstructions do not account for cardiac and respiratory motion; and (III) 3D models are still projected in 2D plane of the visual screen (5). Understanding of the complex pathoanatomy rests on mental reconstruction only aided by a 2D, unisensory visual input.
Although 3D printed anatomical models of an individual patient’s heart remain static, but are reviewed binocularly, they also offer interactivity and hands-on approach. Touching and feeling an object activates the same area in the brain as vision, thus neural responses are modulated by sensory input from other modalities (6). Multisensory convergence enhances visual processing and haptic input significantly contributes to the fine-tuning of the visual information (7). 3D printed models are not to replace reconstructions projected on a screen or into virtual reality but to complement them with tactile and physical element. Thus, personalized imaging and 3D modelling of anatomy presents surgeons with a range of advantages, e.g., better understanding of complex anatomy, a possibility for better preoperative planning and virtual surgery, manufacturing of intraoperative aids and prostheses, ability of assess expected result, improved communication within the multidisciplinary team and with patients (8-10).
3D printing processes, manufacturing of patient-specific prototypes
Technological development brought 3D modelling and printing from the research laboratories into factories and then into people’s homes. It is assumed that 3D printing of medical devices is quick, personalized and could save money. There are two types of 3D printed objects in healthcare: (I) 3D printed anatomical models of an individual patient—an application this article focuses on; and (II) patient-specific medical hardware that also involves computer aided design (CAD)—customized/personalized implants, prostheses, external fixators, splints, surgical instrumentation and surgical cutting aides. Table 1 presents cardiovascular applications are mainly confined to the former area, the models.

Full table
3D printing consists of consecutive steps of digital data acquisition, post-processing [segmentation, conversion of DICOM data into stereolithography (STL) file], production (actual stereolithographic printing, additive manufacturing) and post-production (processes similar to chiselling and refinement in sculpture) (please, also see Supplement I) (Figure 1). First, digital data from imaging sources (CT-angiography, MRI and echocardiography) are obtained. Most commonly ECG-gated breath-held contrast-enhanced CT angiography is used that can provide a spatial resolution of 0.3–0.7 mm. Dataset is processed by a special 3D software (Mimics®, Materialise, Leuven, Belgium) and a rotatable digital (virtual) 3D model is segmented (11). Accuracy of segmentation depends on the completeness and clarity of raw data and appropriate selection of segmentation values. Areas and structures of interest are exposed while others (temporarily) removed. Special refinements are also done for smoothing the model’s surface by a user-defined number of iterations. All this requires intimate knowledge of anatomy, thus close-cooperation between the clinical engineer and clinician/surgeon is advised; segmentation is also time-consuming, laborious and—at present—it is not feasible for automation (9). A virtual model already offers indispensable insight: it can be rotated and cross-sections at any plane and depth can reveal intricate details of anatomy (Figure 2). The actual printing process is rapid prototyping and additive manufacturing, building parts layer by layer. In our clinical practice two prototypes are 3D printed: a real life-sized, blood-volume solid model provides exact dimensions of the anatomical structures; another hollow model (wall model for endocardial and epicardial surface representation) printed in translucent material of which the material properties are within the range of real human arterial tissue properties (Young’s modulus between 0.2 and 9 MPa; distensibility between 1.2×10−3 and 6.6×10−3 mmHg−1). The hollow model can be magnified by 1.5–2.5× in order to help spatial orientation inside the cavities of a small neonatal or infant heart. Translucent, flexible material helps in simulating surgical approach and steps of the operation with high-fidelity (12) (Figure 3).
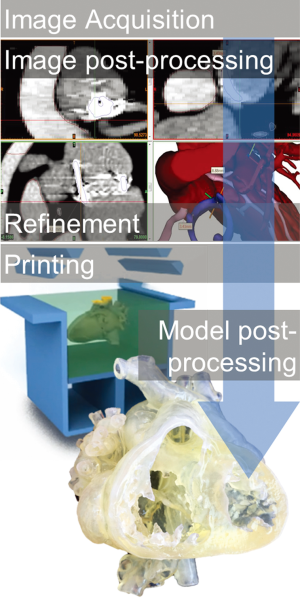
Intraoperative assessment confirms anatomic accuracy of 3D models (Figure 4). Prototyping contributes to improved patient safety and shortened operating time, successful outcome (13). Among the multiple benefits of 3D printed models are the improved communication within the multidisciplinary clinical team and patient/family education (14). Feasibility of new surgical procedures and/or catheter interventions could be experimented with patient-specific morphological characteristics (15). Virtual surgery can also be performed (16,17). Besides listed and documented advantages, 3D printing presents with possible downsides: labour—and technology intensive manufacturing presents with additional costs, needs extra personnel and infrastructure (e.g., 3D printing facility) (18). According to a meta-analysis of 158 papers on 3D printing in surgery, accuracy of the model was only satisfactory in 33.5%; time spent with the production of the model only resulted in equivalent savings in OR time in 32.9% (19).
Applications of 3D modelling and printing in pediatric cardiac surgery
Pediatric cardiac surgery deals with a wide range of patients of age (from neonatal to adult congenital), of acuity (from emergencies to elective and/or staged reoperations), and all complexities. Most operations are performed with a special attention to the expected growth of structures and assumed transformation of pathophysiology. Despite significant advances in surgical technique and perioperative management, our specialty still carries significant risks (20). These aspects predispose pediatric cardiac surgery to early embrace new modalities in the pursuit of patient-safety and high quality-of-care. Indeed, prevalence of modelling for the heart and vessels especially in the congenital cardiac domain is only second to skull/facial 3D printed models (10).
Pediatric cardiac surgery is also a discipline where individual decision-making is the basis of the planning for complex operative scenarios. Better preoperative planning has been demonstrated to associate with shortened intraoperative time that per se has significant impacts on complication rate, blood loss, postoperative length-of-stay, etc. (21). Detailed knowledge of the general pathomorphology is a prerequisite before embarking on any individual surgical procedure. Historically, generations of physicians and surgeons were educated with the help of ex vivo cardiac specimens. Although specimens come from individual patients featuring their own unique cardiac malformations, they represent general features of morphology. Dr. Maude Abbott [1869–1940], founder of pathomorphology for congenital heart disease, began ‘museum demonstrations’ in 1904 that had become part of the medical school curriculum (22,23). In recent years, however availability of these archives has become limited due to stiffened data protection regulations (24), reduced number of autopsies, natural attrition of specimens and most importantly, patients with congenital heart disease survive (25). Source of specimens has dramatically dropped. Transfer of specimens in the morphological archives onto digital platform and creation of a virtual museum from clinical data could solve the problem (26). First, specimens are scanned with high-resolution micro-computed tomography (it can achieve a resolution of 10 micrometres) (27,28). Next, digital information is segmented to create 3D virtual models and could be 3D printed in various materials. A virtual museum offers innumerable opportunities for training and education, pre-surgical planning and virtual surgery, patient-family education, etc. (29).
Introduction of 2D echocardiography enhanced the importance of functional anatomical knowledge in our discipline that is further emphasized by newer imaging modalities. Learning-curve for surgical trainees has become rather steep; no collateral morbidity/mortality is now tolerated. Access to morphological archives—as mentioned—became restricted. Simulation-based methods with 3D (virtual) models and printed prototypes (clinical case scenarios and specimens) could overcome these difficulties and meet the demands of morphological demonstration. Interactivity and hands-on approach is the foundation in modern day medical and postgraduate education (30). Medical education ranges from medical students, trainees, the multidisciplinary clinical team and towards patients/families and the community. For physicians and the healthcare team tactile and binocular input, improved spatial awareness, better understanding of complex pathology, communication and simulation tool for surgical and interventional cases leading to better pre-operative planning are the core benefits (14). Better understanding of one’s conditions translating into more realistic expectations, and therefore, more comfortable with options are among the listed patient benefits. Anecdotal benefits, e.g., reduction in intraoperative time and cost, anaesthesia time, infection rate, radiation and contrast, complications and hospital stay need to be validated in randomized controlled trials. These trials are traditionally difficult to execute in surgery (31,32), especially when the triggering indicator for 3D printing is the uniqueness and complexity of anatomy as well as its rarity. As a critical mass of experience has not yet been accumulated, no conclusion can be drawn how effective 3D prototypes are. Personal experience identifies patient-safety and reduced occurrence of complications and ultimately improved quality of care as major advantage.
Selected case scenarios
Application of 3D models and printed prototypes has become a regular practice in preoperative planning for complex re-operations by many teams worldwide. All case-scenarios presented below alongside the segmental approach have undergone successful and uncomplicated operations. Actual 3D printed models are jointly utilized together with the 3D virtual models as the latter can be opened at any trans-sectional plane, rotated, digitally modified, etc.
Figure 5 demonstrates a case of right atrial isomerism, single left superior vena cava receiving hemiazygos continuity of the interrupted inferior vena cava; common atrium, incomplete AV defect, dextrocardia of the ventricular loop. The patient previously underwent atrial septation and repair of the left AV valve but presented with recurring baffle leak. 3D visualization of the atrial anatomy and connections of the pulmonary and systemic veins in complex atrial baffling procedures offers unique possibility for tailoring geometrically challenging separation patches. By opening the virtual 3D model at any choice of plane, anatomy of dehiscence could be easily revealed. Hands-on exploration of the 3D printed hollow model immediately allowed good understanding and facilitated the feasible surgical approach. With the proper understanding of the anatomy, reoperation could be reduced to an ‘ASD patch closure’.
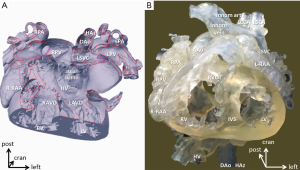
Present technical capabilities of 3D printing do not provide adequate representation of the atrioventricular valves (9). With the refinements of the data resources (e.g., improvement on echo data) (33), and in vitro utilization a micro-computed CT, it is expected that imaging quality of the valves, leaflets improves (34).
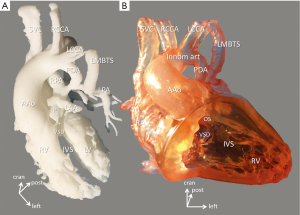
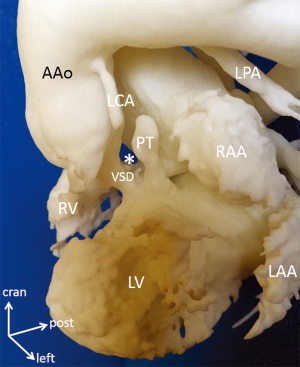
Double outlet right ventricle often mandates complex design of intraventricular tunnelling and muscle resection where 3D modelling and pre-surgical planning has been proven a valuable tool (35,36). Figure 3 presents 3D printed models of a double outlet right ventricle, extreme subpulmonary stenosis, right aortic arch, left-sided patent arterial duct originating from the innominate artery and left modified Blalock-Taussig shunt LMBTS perfusing hypoplastic left pulmonary artery. Blood volume model reveals the severity of subpulmonary obstruction, the extent of hypoplasia of the left pulmonary artery. As a new finding, it is noted that left atrial appendage courses across the intended incision of the pulmonary trunk. Scaled, translucent hollow-model printed in flexible material grants the trial of resecting the outlet septum to create straight connection between the left ventricle and aorta.
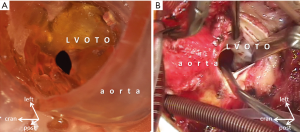
A 6-month-old patient presented with tricuspid atresia, juxtaposed right atrial appendage, restrictive VSD, transposed great arteries, pulmonary atresia, and hypoplasia of the left pulmonary artery (Figure 6). 3D printed blood volume model identifies relationship of the restrictive VSD and outlet septum that permits planning of transaortic subaortic resection without the risk AV block and/or injury of structures. The model reveals high take-off of the left main coronary artery in the area of planned aortotomy. Preoperative exploration of the 3D printed hollow model through the ascending aorta provided excellent view to the subaortic obstruction that was identical with the intraoperative view (Figure 4). Patient underwent uncomplicated relief of the obstruction and patch augmentation of the hypoplastic left pulmonary artery and bidirectional cavopulmonary anastomosis.
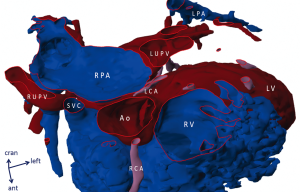
Preoperative evaluation of 3D virtual and printed models often offers special insights into rare coronary abnormalities. Orifice of the left coronary artery is flattened and obstructed by grossly dilated right pulmonary artery in a case of tetralogy of Fallot and absent pulmonary valve syndrome. Initial segment of the left coronary artery is much smaller than its right counterpart. Lecompte manoeuvre is preferred to relieve the compression (Figure 2). Preoperative knowledge of the exact position and course of an aberrant right coronary artery originating from the left descending artery of the left coronary artery is crucial in avoiding injury during the placement of the right ventricle to pulmonary bifurcation conduit (Figure 7). Of course, suspicion about these rare phenomena is raised by other imaging modalities (2D echocardiography, CT, MRI), however 3D virtual and printed models present with unparalleled insight into the intimate spatial relationship of the structures as well as contribute to preoperative planning.
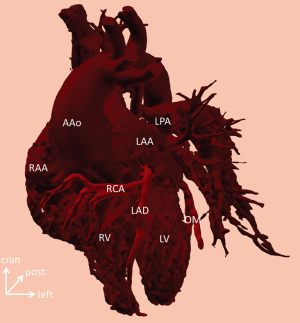
In summary, the use of the 3D virtual and printed models in pediatric cardiac surgery elucidates the anatomy, can add important new findings, and, with the tactile element, helps in pondering the feasibility of different surgical approaches. Non-scaled blood volume models are especially accessible for taking measurements and planning procedures on the great vessels and their branches. Accuracy of the models is excellent, even after moderate postproduction smoothing. Familiarization with non-expected operative anatomy, e.g., high take-off of the left main coronary artery in the area of planned aortotomy, or left atrial appendage crossing the pulmonary trunk can avoid serious complications, adverse surgical scenarios. Prior knowledge, preparedness and possibility of emulation are indispensable in raising the safety boundary.
A centre of excellence (COE) in 3D printing for healthcare
We employ the example of the United Arab Emirates as convenient demonstration for the possible impact of 3D printing. Vibrant and ever-evolving sociocultural and technological context of this country has initiated the establishment of a COE in the field of 3D printing in the construction sector, healthcare and for consumer products (37). We propose five pillars of success of such a venture in the healthcare sector. First and most importantly governmental leadership should embrace and support this rapidly growing and pioneering area by providing transparent legal framework and a spectrum of endowed programs. Programs by invitation may span for specific clinical applications in orthopaedics, maxillofacial surgery, plastic and reconstructive surgery to cardiovascular surgery to prosthetics and development in basic and clinical research of bioscaffolds, bioengineered materials, 3D printed tissues and organs, etc. Secondly, local academic research organizations in biomedical and bioengineering sciences are important in providing scientific leadership and proper prioritization of viable projects. The third pillar is the involvement of clinical healthcare (professionals and providing institutions) where individual projects can find their realization, outcome and continuous feed-back for research. Fourth, healthcare financers should be motivated and involved. 3D printing definitely represents extra costs that may not be justified by reduction in OR-time but improved patient-safety, less complication rate. These are, however, anecdotal benefits difficult to prove statistically. Financial cover for the use of 3D printed models and aids remains unresolved worldwide. At present, there are no internationally established current procedural terminology (CPT) codes available for insurance companies and/or healthcare financial bodies to cover expenses related to 3D printing. Category III codes (established for emerging technology, services and procedures) could only be used; payers, however, may still not approve 3D printed models and devices for the lack of proven benefit demonstrated in randomized controlled trials (38). Finally, the fifth key element is the integration of local 3D printing companies who act as an interface with the world of rapidly-evolving technology are seminal in adapting new methods from 3D printing outside of healthcare. Governance of the COE is based on cooperation and communication among all key participants along a governmental legal framework, established scientific guidelines in research, clinical benefit of the patients, and financial sustainability.
Future prospects
It is expected that 3D printing will have a major role in providing patient-specific (individually customized) implants and prostheses, especially with evolving techniques of bioprinting (39). Bioscaffolds seeded with progenitor cells of the recipient may develop into complex structures, tissues and ultimately organs (40). In cardiac domain, all this could help in fulfilling the ultimate goal to create working myocardium or an ideal—living and growing—cardiac valve implant.
Another new direction of 3D imaging technology is image-guided surgery/augmented reality. With this commercially available modality, patient-specific 3D models or holograms are projected to a fixed point in virtual space (41). 3D image data could be directly superimposed on structures of the operative area, so that key landmarks of the 3D holographic model are identified and paired with counterparts of the patient’s anatomy (42). In combination with robotics, optical display could revolutionize surgery in the future: it could allow procedures in the heart with preserved perfusion/organ function while being operated. The operator performs procedures on the holographic model in the 3D virtual reality and robotic micromanipulators would identically follow the same movements in the patients’ real surgical field (43). Of course, there are myriads of problems to resolve, e.g., interactivity between the holographic model and real organ—as the latter moves and changes shape and size in time with the cardiac cycle that the virtual model should exactly follow—just to mention one. Nevertheless, such prospects in 3D technology revive an intellectual excitement comparable to the one that established anatomy as medical science and paved the way for modern medical and surgical methods 500 years ago.
Supplement I
It all starts with imaging
When creating anatomical models, great imaging is the precursor for good-quality 3D models and an efficient process. CT or MRI? Contrast-enhanced or non-contrast? Each modeling application will have unique imaging requirements, so a close collaboration with the imaging departments is key.
In their review paper, Yoo et al. highlighted a few important factors to take into account regarding imaging used for model creation: (I) high spatial and temporal resolution; (II) the use and amount of contrast agent; and (III) the timing of the scanning after applying the agent (9).
Image processing and 3D modeling
The software used to convert the images to a 3D visualization allows to combine automated and manual segmentation, while rendering the end result on the spot. This gives you the advantage of seeing and verifying what you have created prior to 3D printing. It goes without saying that knowledge in imaging and anatomy/pathology is advantageous for accurate segmentation.
After segmenting the images, further preparation and augmentation of the segmented anatomy is done. In order to enhance visualization of intra-cardiac anatomies for example, windows can be provided and different colors can be used. In cardiology applications, two types of models are found to be valuable, depending on the application: cast models for blood pool representation and wall models for endocardial surface representation (9).
Prior to printing, it is recommended to verify the contours of the resulting model against the original DICOM images in the software. As a final step, pre-labeling the model in the software can be done to allow for an easy identification. When ready, the reconstruction can now be exported now as a printable file.
Depending on the needs and budget, different materials and technologies can be used to print the model in (e.g., Polyjet, SLS and SLA), all with their unique advantages for certain applications.
Additionally, to exporting 3D printable files, the Mimics Innovation Suite software is also able to export 3D PDFs of the created model. This 3D PDF is easy to use and can be opened in any standard 3D PDF viewer available. Great features of this PDF are the ability to render the shown model transparent or switch parts on and off.
Acknowledgements
The author acknowledges the contribution of Ms. Magali Minet and Mr. Carlos Perez, Clinical Engineers from Materialise, Leuven, Belgium who superbly performed all postprocessing, segmentation and production of the models. Ms. Magali Minet is also the author of Supplement I.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Rippa-Bonati M. Some traditions regarding the old anatomy theatre of Padua University. Retrieved 6 May 2016. Available online: http://www.unipd.it/esterni/visiteweb/english/pagine/teatro.htm
- Ghosh SK. Evolution of illustrations in anatomy: a study from the classical period in Europe to modern times. Anat Sci Educ 2015;8:175-88. [Crossref] [PubMed]
- Fagan TE, Truong UT, Jone PN, et al. Multimodality 3-Dimensional Image Integration for Congenital Cardiac Catheterization. Methodist Debakey Cardiovasc J 2014;10:68-76. [Crossref] [PubMed]
- Kurup HK, Samuel BP, Vettukattil JJ. Hybrid 3D printing: a game-changer in personalized cardiac medicine? Expert Rev Cardiovasc Ther 2015;13:1281-4. [Crossref] [PubMed]
- Biaggi P, Fernandez-Golfín C, Hahn R, et al. Hybrid Imaging During Transcatheter Structural Heart Interventions. Curr Cardiovasc Imaging Rep 2015;8:33. [Crossref] [PubMed]
- James TW, Humphrey GK, Gati JS, et al. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia 2002;40:1706-14. [Crossref] [PubMed]
- Lunghi C, Alais D. Touch Interacts with Vision during Binocular Rivalry with a Tight Orientation Tuning. PLoS One 2013;8:e58754. [Crossref] [PubMed]
- Mottl-Link S, Boettger T, Krueger JJ, et al. Images in cardiovascular medicine. Cast of complex congenital heart malformation in a living patient. Circulation 2005;112:e356-7. [Crossref] [PubMed]
- Yoo SJ, Thabit O, Kim EK, et al. 3D printing in medicine of congenital heart diseases. 3D Printing in Medicine 2016;2:3-15.
- Hoang D, Perrault D, Stevanovic M, et al. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med 2016;4:456. [Crossref] [PubMed]
- Mimics®, Materialise, Leuven. Retrieved on 20 September 2016. Available online: http://www.materialise.com/
- Kiraly L, Tofeig M, Jha NK, et al. Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair. Interact CardioVasc Thorac Surg 2016;22:238-40. [Crossref] [PubMed]
- Schmauss D, Haeberle S, Hagl C, et al. Three-dimensional printing in cardiac surgery and interventional cardiology: a single-centre experience. Eur J Cardiothorac Surg 2015;47:1044-52. [Crossref] [PubMed]
- Biglino G, Capelli C, Wray J, et al. 3D manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ Open 2015;5:e007165. [Crossref] [PubMed]
- Schievano S, Migliavacca F, Coats L, et al. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 2007;242:490-7. [Crossref] [PubMed]
- Shiraishi I, Yamagishi M, Hamaoka K, et al. Simulative operation on congenital heart disease using rubber-like urethane stereolithographic biomodels based on 3D datasets of multislice computed tomography. Eur J Cardiothorac Surg 2010;37:302-6. [PubMed]
- Ong CS, Loke YH, Opfermann J, et al. Virtual Surgery for Conduit Reconstruction of the Right Ventricular Outflow Tract. World J Pediatr Congenit Heart Surg 2017;8:391-3. [Crossref] [PubMed]
- Hibino N. Three Dimensional Printing: Applications in Surgery for Congenital Heart Disease. World J Pediatr Congenit Heart Surg 2016;7:351-2. [Crossref] [PubMed]
- Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery 2016;159:1485-500. [Crossref] [PubMed]
- Coulson JD, Seddon MR, Readdy WF. Advancing safety in pediatric cardiology – approaches developed in aviation. Congenital Cardiology Today 2008;6:1-10.
- Maruthappu M, Duclos A, Lipsitz SR, Orgill D, et al. Surgical learning curves and operative efficiency: a cross-specialty observational study. BMJ Open 2015;5:e006679. [Crossref] [PubMed]
- Maude Abbott. Retrieved on 24 September 2016. Available online: http://www.mcgill.ca/medicalmuseum/introduction/history/physicians/abbott
- Abbott ME. Atlas of Congenital Cardiac disease. New York: The American Heart Association, 1936.
- Smith R. All changed, changed utterly. British medicine will be transformed by the Bristol case. BMJ 1998;316:1917-8. [Crossref] [PubMed]
- Wren C, O’Sullivan JJ. Survival with congenital heart disease and need for follow up in adult life. Heart 2001;85:438-43. [Crossref] [PubMed]
- Mostefa-Kara M. 3D imaging of heart specimens: a new teaching tool for understanding the anatomy of double outlet right ventricle. Retrieved on 16 June 2016. Available online: http://www.uni-kiel.de/aepc/2016/aepcAbstractsFinalPrint/O4_6fin.pdf
- Happel CM, Klose C, Witton G, et al. Non-destructive, high-resolution 3-dimensional visualization of a cardiac defect in the chick embryo resembling complex heart defect in humans using micro-computed tomography: double outlet right ventricle with left juxtaposition of atrial appendages. Circulation 2010;122:e561-4. [Crossref] [PubMed]
- Hutchinson JC, Shelmerdine SC, Simcock IC, et al. Early clinical applications for imaging at microscopic detail: microfocus computed tomography (micro-CT). Br J Radiol 2017;90:20170113. [Crossref] [PubMed]
- Preece D, Williams SB, Lam R, et al. "Let's get physical": advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat Sci Educ 2013;6:216-24. [Crossref] [PubMed]
- Baker CJ, Sinha R, Sullivan ME. Development of a cardiac surgery simulation curriculum: from needs assessment results to practical implementation. J Thorac Cardiovasc Surg 2012;144:7-16. [Crossref] [PubMed]
- Hardin WD Jr, Stylianos S, Lally KP. Evidence-based practice in pediatric surgery. J Pediatr Surg. 1999;34:908-12; discussion 912-3. [Crossref] [PubMed]
- Mansouri A, Cooper B, Shin SM, et al. Randomized controlled trials and neurosurgery: the ideal fit or should alternative methodologies be considered? J Neurosurg 2016;124:558-68. [Crossref] [PubMed]
- Bartel T, Rivard A, Jimenez A, et al. Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur Heart J 2018;39:1246-54. [Crossref] [PubMed]
- Lombardi CM, Zambelli V, Botta G, et al. Postmortem microcomputed tomography (micro-CT) of small fetuses and hearts. Ultrasound Obstet Gynecol 2014;44:600-9. [Crossref] [PubMed]
- Ma XJ, Tao L, Chen X, et al. Clinical application of three-dimensional reconstruction and rapid prototyping technology of multislice spiral computed tomography angiography for the repair of ventricular septal defect of tetralogy of Fallot. Genet Mol Res 2015;14:1301-9. [Crossref] [PubMed]
- Yoo SJ, van Arsdell GS. 3D Printing in Surgical Management of Double Outlet Right Ventricle. Front Pediatr 2018;5:289. [Crossref] [PubMed]
- Available online: http://www.dubaifuture.gov.ae/?s=3D+printing+strategy, retrieved on 26 February 2018.
- Available online: https://www.aapc.com/resources/medical-coding/cpt.aspx, retrieved on 3 November 2017.
- Turksen K. editor. Bioprinting in Regenerative Medicine. 1st ed. Springer International Publishing, Switzerland, 2015.
- Markovic M, Van Hoorick J, Hölzl K, et al. Hybrid Tissue Engineering Scaffolds by Combination of Three-Dimensional Printing and Cell Photoencapsulation. J Nanotechnol Eng Med 2015;6:0210011-7. [Crossref] [PubMed]
- RealViewHearth. Surgery using 3D hologram. Published on 11 Nov 2013, retrieved on 10 September 2016. Available online: https://www.youtube.com/watch?v=K2XesWsL9UY
- Oktay O, Bai W, Guerrero R, et al. Stratified Decision Forests for Accurate Anatomical Landmark Localization in Cardiac Images. IEEE Trans Med Imaging 2017;36:332-42. [Crossref] [PubMed]
- Hurley J. Building robots for the cutting edge of medicine. A Cambridge-based company is intent on leading a ‘revolution in surgery’. The Times, 16 August 2017, retrieved on 3 November 2017. Available online: https://www.thetimes.co.uk/past-six-days/2017-08-16/business/building-robots-for-the-cutting-edge-of-medicine-nhnk5c65j#


