Nonoliguric hyperkalemia in a late preterm infant with severe birth asphyxia
Although hyperkalemia is a common and serious complication among preterm neonates, it is usually in extremely premature infants. Nonoliguric hyperkalemia (NOHK) has been defined as serum potassium 7 mmol/L during the first 72 hours of life in the presence of urinary output of >1 mL/kg.h (1). It was reported that more than 80% of babies with NOHK were ELBW infants with gestational age below 28 weeks (2). Few cases have been reported in late preterm infants with NOHK who were born at 34 0/7 to 36 6/7 weeks’ gestation. We therefore report a case of a 3-hour-old late preterm infant who was born with severe birth asphyxia and developed NOHK at 12 hours age. The neuroimaging finding of the infant was showed as well.
Case report
A premature baby boy was delivered at 34 weeks and 3 days’ gestational age. He was delivered by cesarean section for failing to vaginal delivery. Apgar scores were 4 and 5 at 1 and 5 minutes, respectively. No abnormal placenta, umbilical cord and amniotic fluid were found. Birth weight was 2,210 g. The baby developed grunt and cyanosis right after birth and was no any relief after oxygen inhalation. No seizures were noticed. No blood transfusion or medications was received. He was transported to our hospital 3 hours later. Body temperature was 36.5 °C on admission. His pulse and respiratory rates were 150 beats and 66 per minute, respectively. Oxygen saturation (SpO2) was 70% without oxygen. He was awake but hyporeactive with irritability and sharp crying, tachypnoea and mild flaring of the alae nasi. Anterior fontanel was normotensive. There was a cephalohematoma (5 cm × 5 cm) on the right top of the head. The respiratory movements were fast and shallow. Lungs were clear to auscultation. The heart rate was regular and no murmurs were heard. The heart sound was strong. The abdomen was soft on palpation, and no hepatosplenomegaly. Borborygmus and umbilical cord were normal. He showed poor peripheral perfusion with chilly and capillary refilling time 4 seconds. Muscle tension was reduced on the upper limbs and normal on the lower limbs. Hugging and holding reflexes were incomplete, whilst foraging, sucking and swallowing reflexes were all disappeared. Gestational age assessment was 34 weeks.
The baby was sedated with phenobarbital in the first day after admission. Oxygen was administered by face mask at 1 L/min in the first two days for the relief of cyanosis. Serum potassium reached to 8.3 mmol/L at 12 hours age, and meanwhile the baby developed abnormal ECG of tall peaked T waves with 134 bpm of the heart rate. Calcium gluconate 10%, 1 mL/kg diluted 10 folds with glucose 5%, i.v. bolus at 1 mL/min was employed. Insulin and glucose (1 unit to 4 g) were infused at 0.05 units/kg.h. Urinary output was monitored closely which was between 2.41 to 2.64 mL/kg.day and blood glucose was normal during hospitalization. Serum potassium came down gradually below 6.5 mmol/L at 28 hours age. Laboratory findings showed normal white blood cells, and 247×109/L of platelets, no anemia. Two serum urea and creatinine were 3.89 mmol/L and 74.5 µmol/L, 4.32 mmol/L and 70 µmol/L, respectively. C-reactive protein <1.00 mg/L. TORCH-IgM were negative and blood culture was sterile. Marked fall in serum Ca2+ of 0.6 mmol/L and total calcium of 1.13 mmol/L (Table 1) at age of 37 hours was noted, accompanied by weak heart sound and bradycardia (115/min). Serum inorganic phosphorus was 3.91 mmol/L, 0.78 mmol/L of magnesium (0.67-1.15 mmol/L) and 158 U/L of alkaline phosphatase. A 10% solution of calcium gluconate (2 mL/kg) was infused at the same dilution as above. Ionized calcium increased to 1 mmol/L on the 5th day after birth. Parathyroid hormone (PTH) was 145.0 pg/mL. Blood gas and electrolytes at different time of age were listed as Table 1. Chest X-ray showed mild decreased densities with patchy opacity areas in both lung fields. The head magnetic resonance imaging (MRI) findings were shown (Figure 1).
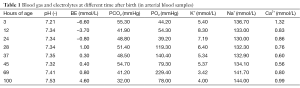
Full table
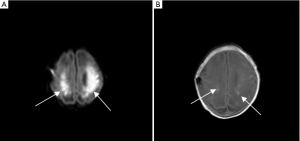
The baby was sedated with phenobarbital for 24 hours after admission. Oxygen was administered by face mask at 1 L/min in the first two days for the relief of cyanosis. The serum potassium increased to 8.3 mmol/L at 12 hours age, and abnormal ECG of tall peaked T waves was noted. The heart rate was 134 bpm. Calcium gluconate 10%, 1 mL/kg diluted 10 folds with glucose 5%, i.v. bolus at 1 mL/min was employed. Insulin and glucose (1 unit to 4 g) were infused at 0.05 units/kg·h. Blood glucose was normal during hospitalization. Urinary output was monitored closely which was between 2.41 to 2.64 mL/kg·day. Serum potassium came down gradually below 6.5 mmol/L at 28 hours of age. Laboratory findings showed normal white blood cells, and 247×109/L of platelets, no anemia. Two serum urea and creatinine were 3.89 mmol/L and 74.5 µmol/L, 4.32 mmol/L and 70 µmol/L, respectively. C-reactive protein <1.00 mg/L. TORCH-IgM were negative and blood culture was sterile. Marked fall in serum Ca2+ of 0.6 mmol/L and total calcium of 1.13 mmol/L (Table 1) at age of 37 hours was noted, accompanied by weak heart sound and bradycardia (115/min). Serum inorganic phosphorus was 3.91 mmol/L, 0.78 mmol/L of magnesium (0.67-1.15 mmol/L) and 158 U/L of alkaline phosphatase. A 10% solution of calcium gluconate (2 mL/kg) was infused at the same dilution as above. Ionized calcium increased to 1.0 mmol / L on the 5th day of age. Parathyroid hormone (PTH) was 145.0 pg/mL. Blood gas and electrolytes at different time of age were listed as Table 1. Chest X-ray showed a mild decreased densities with patchy opacity areas in both lung fields. The head magnetic resonance imaging (MRI) findings were shown (Figure 1). Extensive brain damage was showed with abnormal high signal in periventricular white matter. The baby was stable in the following days and discharged at the age of 18 days.
Discussion
In 1977, Perkkiö and Räihä reported a single episode of hyperkalaemia at the age of 17 hrs in an otherwise healthy premature infant, representing the first description of a clinical entity now referred to as nonoliguric hyperkalaemia of the premature infants (3,4). Nonoliguric hyperkalemia (NOHK) has been defined as serum potassium >7.0 mmol/L during the first 72 hours of life in the presence of urinary output of >1 mL/kg·h. It is more common in extremely low birth weight infants than term infants and the reported incidence varies widely between 11% and 52%. This reversible condition does not appear to be related to renal failure, increased potassium intake, or excessive bruising (1,2,5,6). It was significantly associated with fetal distress, early metabolic acidosis, early hyperglycemia, and absence of antenatal steroid administration (2). A study by Yaseen et al. (2) and Kazuo et al. (7) demonstrated that more than 80% of babies with NOHK were extremely low birth weight infants with gestational age <28 weeks, because the potassium balance for premature infants with gestational age 33 to 36 weeks was more stable than those under 33 weeks. In this paper, we reported a late preterm infant with NOHK which were no reports before. Late preterm is the recommended definition for infants born at 34 0/7 to 36 6/7 weeks’ gestation. The baby was born with severe birth asphyxia and developed hyperkalemia at 12 hours age. Urinary output as well as serum urea and creatinine were all at normal levels.
The mechanism of NOHK is suggested mainly an immature function of Na/K ATPase activity in severe prematurity resulting in an internal potassium from intracellular to plasma. Fetal distress, low Apgar scores, and early neonatal acidosis or glucose and insulin abnormality were all significantly associated with the high incidence of potassium transport towards the intracellular space decreased and intracellular potassium into the extracellular space by leakaging or shifting (6,8,9). Some people found that the complete inactivation of aldosterone could result in hyperkalemia, which was frequently accompanied by hyponatremia (10). Kazuo et al. (7) thought that in vivo the shift or leakage of potassium was the main factor causing NOHK and was negatively associated with gestational age and postnatal age.
We found in our case a decreased serum calcium as serum potassium decreased which did not change at the same time, and higher serum phosphorous was also noted. Thayyil et al. (11) found that the abnormality of serum calcium was earlier than that of serum potassium in very low birth weight infants with NOHK. Fukuda et al. (12) found that hypocalcemia was more common in preterm infants who had serum potassium >7 mmol/L. The pathogenesis of combination of hypocalcemia and increased serum phosphate may raise the possibility of a transient hypoparathyroidism (2). Whereas PTH was at normal level in our case. We think that it is unlikely that all biochemical abnormalities were merely a coincidence. Associated risk factors for NOHK in late preterm infants have not been identified. In this case, the baby had no extra potassium intake and blood transfusion before the onset of hyperkalemia. Serum sodium was at normal level. Severe birth asphyxia was the only factor for the development of NOHK in this case (13). We think that the biochemical abnormalities in this baby were due to decreased potassium transport towards the intracellular space resulted from birth asphyxia, but in a study by Thayyil et al. (11), no any association with Apgar scores or gestation with hyperkalemia was found. Asphyxia might also influence the activity of calcium related enzyme on cell membrane. This has not previously been reported in the literature and might be useful in recognizing late preterm infants likely to be developing NOHK.
Neonatal hyperkalemia could result in cardiac arrhythmias and has been associated with the development of periventricular leukomalacia, brain hemorrhage and sudden death (7). The head MRI (Figure 1) showed abnormal high signal in periventricular white matter, and it was much more obvious in DWI than in T1WI which predicts poor outcome. Rutherford et al. (14) recommended that DWI is a clinically useful for the early identification of ischaemic tissue in the neonatal brain.
As neonatal hypoxia and neonatal hypoglycemia all contribute to brain injury, one should separate the injuries of the two factors on the basis of the imaging appearance. Barkovich et al. (15) found that the pattern of brain damage in neonatal hypoglycemia was mainly localized primarily to the parietal and occipital cortex of the brain, whereas the common types of injury in the preterm brain from ischemia are intraventricular hemorrhage (IVH) and periventricular white matter injury. As no hypoclycemia was recorded, we speculate that abnormal neurologic activities on admission and brain MRI findings of the baby were due to severe birth asphyxia which resulted in the disturbance of electrolytes in the following days.
The treatment of hyperkalemia in infants varies among institutions and limited information from small studied of uncertain quality on therapeutic for intervention of NOHK. The treatment indication for preterm infants with NOHK is not unified at present. Chevalier (16) thought that therapy should be initiated when serum potassium was greater than 7 mmol/L in a non-hemolysed arterial or venous blood sample, because the risk of cardiac arrhythmia in non-oliguric hyperkalemia of premature infants increased substantially at this level. Serum potassium below 7 mmol/L in the presence of characteristic electrocardiogram changes was also an indication for therapy. Whilst most clinical experience suggests that serum potassium above 6.5 mmol/L should begin treatment regardless of the electrocardiogram changes. Based on our experience, insulin with glucose in the absence of arrhythmia was used if serum potassium was greater than 6.5 mmol/L. Calcium gluconate was admitted if characteristic electrocardiogram developed. Mildenberger et al. (6) prefered causal treatment rather than administration of sodium bicarbonate for the relief of metabolic acidosis. The limitations of this report were that the creatinine clearance and functional excretion of Na and K were not calculated to understand the renal function.
Concerning for the prevention of NOHK, it was reported that antenatal glucocorticoid could promote Na/K-ATP enzyme maturation and could effectively prevent premature with NOHK (17,18), whilst it was unclear in infants resulted from birth asphyixia.
In conclusion, NOHK mainly affects very low birth weight or extremely low birth weight infants and is associated with other electrolyte disturbances with high morbidity and mortality. NOHK in term or late preterm infants was rarely reported. A future study with larger samples is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vemgal P, Ohlsson A. Interventions for non-oliguric hyperkalaemia in preterm neonates. Cochrane Database Syst Rev 2007;1:CD005257. [PubMed]
- Brion LP, Schwartz GJ, Campbell D, et al. Early hyperkalaemia in very low birthweight infants in the absence of oliguria. Arch Dis Child 1989;64:270-2. [PubMed]
- Yaseen H. Nonoliguric hyperkalemia in neonates: a case-controlled study. Am J Perinatol 2009;26:185-9. [PubMed]
- Perkkiö M, Räihä N. Neonatal hyperkalaemia. Lancet 1977;2:143. [PubMed]
- Gruskay J, Costarino AT, Polin RA, et al. Nonoliguric hyperkalemia in the premature infant weighing less than 1000 grams. J Pediatr 1988;113:381-6. [PubMed]
- Mildenberger E, Versmold HT. Pathogenesis and therapy of non-oliguric hyperkalaemia of the premature infant. Eur J Pediatr 2002;161:415-22. [PubMed]
- Sato K, Kondo T, Iwao H, et al. Internal potassium shift in premature infants: cause of nonoliguric hyperkalemia. J Pediatr 1995;126:109-13. [PubMed]
- Stefano JL, Norman ME, Morales MC, et al. Decreased erythrocyte Na+,K(+)-ATPase activity associated with cellular potassium loss in extremely low birth weight infants with nonoliguric hyperkalemia. J Pediatr 1993;122:276-84. [PubMed]
- Lang K. K metabolism. In: Greger R, Windhorst U. eds. Comprehensive human physiology, vol 2. From cellular mechanisms to integration. NewYork: Springer, Berlin Heidelberg, 1996:1586-7.
- Shaffer SG, Kilbride HW, Hayen LK, et al. Hyperkalemia in very low birth weight infants. J Pediatr 1992;121:275-9. [PubMed]
- Thayyil S, Kempley ST, Sinha A. Can early-onset nonoliguric hyperkalemia be predicted in extremely premature infants? Am J Perinatol 2008;25:129-33. [PubMed]
- Fukuda Y, Kojima T, Ono A, et al. Factors causing hyperkalemia in premature infants. Am J Perinatol 1989;6:76-9. [PubMed]
- Shortland D, Trounce JQ, Levene MI. Hyperkalaemia, cardiac arrhythmias, and cerebral lesions in high risk neonates. Arch Dis Child 1987;62:1139-43. [PubMed]
- Rutherford M, Malamateniou C, McGuinness A, et al. Magnetic resonance imaging in hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:351-60. [PubMed]
- Barkovich AJ, Ali FA, Rowley HA, et al. Imaging patterns of neonatal hypoglycemia. AJNR Am J Neuroradiol 1998;19:523-8. [PubMed]
- Chevalier RL. What are normal potassium concentrations in the neonate? What is a reasonable approach to hyperkalemia in the newborn with normal renal function? Semin Nephrol 1998;18:360-1. [PubMed]
- Omar SA, DeCristofaro JD, Agarwal BI, et al. Effect of prenatal steroids on potassium balance in extremely low birth weight neonates. Pediatrics 2000;106:561-7. [PubMed]
- Uga N, Nemoto Y, Ishii T, et al. Antenatal steroid treatment prevents severe hyperkalemia in very low-birthweight infants. Pediatr Int 2003;45:656-60. [PubMed]


