Antegrade cerebral perfusion at 25 °C for arch reconstruction in newborns and children preserves perioperative cerebral oxygenation and serum creatinine
Introduction
Complex aortic arch reconstruction in neonates and children is performed typically under deep hypothermic circulatory arrest (DHCA). This approach has enabled successful outcomes over many decades (1), with cerebral protection achieved by reducing brain metabolism and oxygen requirements. The risk of injury associated with DHCA is not clear, although long periods have been associated with seizures and choreoathetosis (2,3). Long-term neurological complications may manifest as impaired neurodevelopment, with the worst outcomes being observed in newborns with complex congenital heart lesions in need for aortic arch reconstruction under prolonged periods of DHCA (2,4-7). With the intent of maximizing cerebral protection, surgical and perfusion strategies have been developed to selectively perfuse the brain during these operations.
Antegrade cerebral perfusion (ACP) at deep hypothermia emerged as an adjunctive perfusion strategy to DHCA aiming to minimize the use of circulatory arrest and offer additional cerebral protection during arch operations. During ACP, blood flow is supplied to the brain selectively during the critical period of arch reconstruction, while at least partial somatic flow is achieved through collaterals. Somatic ischemia is theoretically lessened during arch reconstruction and the risks of neurological and cognitive deficits following operation are presumably reduced (8,9). With increased experience with ACP in the field of adult aortic arch reconstruction, a more recent evolution from deep hypothermia toward the use of warmer temperatures has occurred (10-12).
The use of tepid temperatures for ACP potentially may reduce the deleterious effects associated with deep hypothermia and rewarming (13). But this cannot be at the expense of cerebral and somatic protection. In the absence of a standardized nomenclature, a recent consensus panel categorized the temperatures into ‘deep’ for a nasopharyngeal temperature of 14.1–20 °C, ‘moderate’ for 20.1–28 °C and ‘mild’ for 28.1–34 °C (14). Mild-moderate hypothermia with ACP is now utilized widely in adults, and although not supported by formal and prospective neurocognitive outcomes data, appears to be a safe and effective strategy for both neurological and somatic protection for periods of less than 60 minutes (10,15,16). In newborns and infants, extended end-to-end repair of coarctation is performed routinely at near-normothermia with all cerebral and systemic perfusion achieved via the innominate artery for periods of approximately 20 minutes, without clinically significant neurological or end-organ injury (17). Notwithstanding, few reports evaluate the use of moderate hypothermia for ACP in neonates and children undergoing aortic arch reconstructions (11,12,18-20).
To this end, our specific aim was to further assess the perioperative impact of ACP at 25 °C on cerebral oxygenation and serum creatinine in newborns and children undergoing arch reconstructions. Herein, we report our experience and outcomes.
Methods
Institutional Review Board approval was obtained for this retrospective study and patient/parent consent was waived. Between 2010 and 2014, 61 patients less than 5 years of age underwent complex aortic arch operation using moderate hypothermia with ACP (40–60 mL/kg/min) and a pH-stat blood gas management strategy. The medical records were reviewed for demographics, preoperative diagnosis, and perioperative course. The patients were categorized into three groups: Stage I or Norwood-type operations (Stage I), isolated aortic arch reconstructions (Arch), and aortic arch reconstructions with other major cardiac procedures (Arch++). Patients with obstructed pulmonary venous return were excluded from this study.
Surgical technique
All operations were performed using a physiologic blood-prime followed by cooling with full-flow cardiopulmonary bypass (CPB) (150 mL/kg/min) using a 6 °C temperature gradient to moderate hypothermia (25 °C). A pH-stat blood gas management strategy, pO2 of 150 mmHg, and hematocrit of 30% were maintained. ACP was delivered via the innominate artery or equivalent with flow rates of 40–60 mL/kg/min, maintaining a mean arterial pressure appropriate for the age of the child (25–55 mmHg). During ACP, the arch branches and descending thoracic aorta were controlled with snares or fine clamps to maintain a bloodless field and maintain cerebral and systemic perfusion pressure. Upon completion of the reconstruction, de-airing, and removal of snares or clamps, ACP was followed by re-warming with full-flow CPB at a maximum gradient of 6 °C.
Cerebral and somatic oxygenation monitoring
Bilateral cerebral and single somatic oximetry were monitored continuously and recorded by near-infrared spectroscopy (NIRS) (Somanetics, INVOS 5100C, Covidien) in all patients, both intraoperatively and postoperatively for 120 hours or until discharge from the intensive care unit (ICU). The non-invasive NIRS probe measures the regional oxygen saturation (rSO2) as a percentage on a scale from 15% to 95%. The probes were placed on both sides of the forehead for cerebral (left and right) readings, and over the right flank for somatic rSO2 readings. For this study, the data were recorded at the following time points: baseline (before CPB), start of CPB, cooling, aortic cross-clamping, start of ACP, during ACP, end of ACP, un-clamping, re-warming, end of CPB, and postoperatively for 120 hourly intervals.
Clinical outcomes and serum creatinine
The intraoperative variables assessed were CPB time, aortic cross-clamp time, ACP flow and time, and lactate levels. Serum creatinine and lactate levels were recorded preoperatively and postoperatively on a daily basis until hospital discharge. Postoperative variables analyzed included postoperative length of ICU and hospital stay, need for extracorporeal membrane oxygenation (ECMO), need for postoperative peritoneal dialysis or dialysis, need for gastrostomy tube, neurological complications (seizures, neurological deficit and stroke), and discharge mortality.
Statistical analysis
Data are shown as mean ± standard deviation (SD), median and range (minimum, maximum), or N (%). Given the number of patients and low incidence of complications, additional statistical analysis was not meaningful clinically or statistically.
Results
Patient characteristics
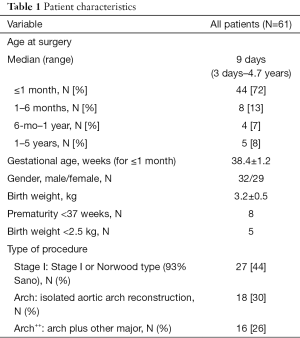
The characteristics for all 61 patients are outlined in Table 1. Median age at surgery was 9 days, with 72% being neonates and 20% infants between 1 month and 1 year of age. Thirty-two patients were male. Among the three groups analyzed, 27 patients (44%) underwent a Norwood-type (Stage I) operation for hypoplastic left heart syndrome (HLHS) or single ventricle variants with arch hypoplasia [unbalanced atrioventricular canal, truncus arteriosus with hypoplastic arch, transposition of the great arteries (TGA) with hypoplastic arch, or interrupted arch]. Of these, 25/27 (93%) Stage I operations received a right ventricle-to-pulmonary artery shunt (Sano). In the second group, eighteen patients (30%) underwent isolated reconstruction of the aortic arch (Arch). In the third group (Arch++), sixteen (26%) patients underwent aortic arch reconstruction along with other major procedures such as a Damus-Kaye-Stansel reconstruction with bidirectional Glenn (Comprehensive stage II), subaortic resection, ventricular septal defect closure, aortic/truncal root replacement, or supravalvular aortic stenosis repair.
Full table
Operative outcomes
The operative outcomes are summarized in Table 2. All aortic arch operations were performed at a mean rectal temperature of 25.0±0.9 °C. Mean CPB and aortic cross-clamp times for all sixty-one patients were 195±95 and 87±61 min, respectively. ACP was performed at a mean flow rate of 46±6 mL/min/kg for 52±22 minutes.
Full table
Cerebral and somatic oxygenation
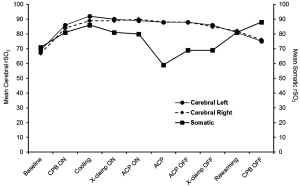
The cerebral and somatic NIRS (rSO2) readings are shown in Figures 1-3. Cerebral NIRS readings stayed above baseline throughout surgery, with no clinically-significant differences in the intraoperative NIRS readings between the left and right cerebral hemispheres for all patients (Figure 1). Somatic NIRS stayed above baseline during cooling, dropped somewhat during ACP, and rebounded quickly after ACP.
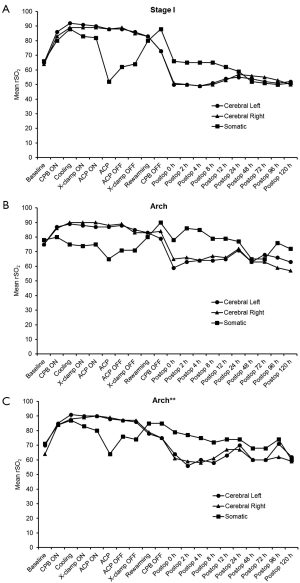
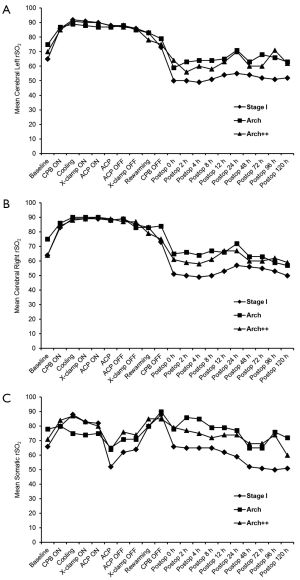
Postoperatively, cerebral and somatic NIRS remained near or at baseline during the first 24 hours and beyond for all groups (Figures 2,3).
Postoperative course
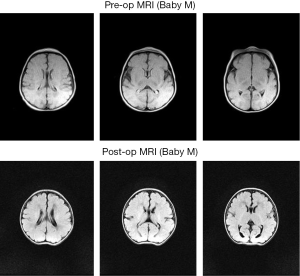
The postoperative outcomes for all 61 patients and by procedure group are described in Table 3. Of the 61 patients, a total of 6 (9.8%) required ECMO. Three were in the Stage I (Norwood) group, and the other three had Arch++ procedures. Median postoperative lengths of hospital and ICU stay for all sixty-one patients were 16 days (range, 4–104 days) and 9 days (range, 1–104 days), respectively. Two patients in the Stage I group received temporary peritoneal dialysis postoperatively for fluid removal. No patient required hemodialysis. None of the patients demonstrated evidence of liver dysfunction. Three patients (4.9%) had an isolated seizure after surgery, two of which were confirmed by electroencephalogram. None persisted after initiation of medical therapy. None of the patients had a neurologic deficit or stroke. Although not the focus of this study, representative pre- and post-operative brain MRI imaging is demonstrated in Figure 4.
Full table
Serum creatinine
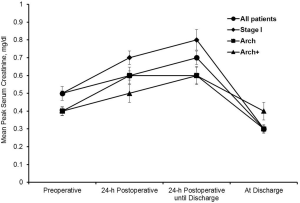
The mean of the peak serum creatinine levels is shown in Figure 5. The peak creatinine for all patients averaged 0.7±0.3 mg/dL. The highest postoperative creatinine of any single patient was 1.48 mg/dL.
Discharge mortality
Overall, there were four discharge mortalities (6.6%). One patient underwent Stage I with a 3.5-mm modified Blalock-Taussig shunt. After an uneventful postoperative course and chest closure, the patient was placed on ECMO on postoperative day (POD) 6 for respiratory distress and ultimately expired on POD 55. The second patient underwent late stage I with Sano after presenting at 6 weeks of age. Despite a favorable neurological and hemodynamic result, the child died of chronic respiratory failure on POD 104. The third patient underwent Stage I and interrupted aortic arch repair. Initially the child did well neurologically and hemodynamically but was placed on ECMO on POD 4 for sudden cardiac arrest. During ECMO wean, the circuit clotted acutely and the child died on POD 8. The fourth patient underwent redo truncal valve replacement and arch reconstruction. The patient was placed on post-operative ECMO for bleeding and inability to separate from CPB from pulmonary dysfunction. The child separated from ECMO but ultimately expired on POD 35.
Discussion
Deep hypothermic circulatory arrest is the traditional approach for operations involving aortic arch reconstruction in adults and children, acknowledging the potential for neurological complications including cognitive deficits. The transition from the DHCA paradigm toward ACP with deep hypothermia was aimed to maximize cerebral protection during arch operations while minimizing any morbidity. Antegrade cerebral perfusion is used now by many centers as a perfusion adjunct under deep hypothermia to minimize the use of circulatory arrest during neonatal aortic arch reconstruction (21), with the expectation of mitigating neurological and somatic morbidity. A comparison of DHCA alone versus continuous low-flow cerebral perfusion in infants has suggested more neurological perturbations and a greater likelihood of clinical seizures in the early postoperative period of the DHCA alone group (3). Other reports advocate the use of ACP over DHCA alone to not only attenuate neurological morbidity but also to achieve somatic protection during arch reconstruction (5,22-25). However, other reports question the advantage of ACP over DHCA alone, detecting no difference in the incidence of new white matter injury or cerebral ischemic lesions postoperatively, nor any benefit on psychomotor and mental development status between the two groups of ACP versus DHCA alone (26-30).
It is worth mentioning that even though ACP is used routinely in many centers, there exist wide variations in the specific details of the perfusion strategy. ACP flow rates, blood gas temperature correction (pH versus alpha stat), time required for the repair, hematocrit, pO2, and even cannulation strategies vary significantly, making it challenging to evaluate the benefit of cerebral perfusion during arch repairs. Despite the lack of a standardized protocol for ACP and some inconsistency in the reported results, there does appear to be an increasing trend toward ACP (with deep hypothermia) over DHCA for neonatal arch reconstruction (31).
The optimal temperature for complex aortic arch reconstructions with ACP remains a topic of debate. Many adult centers have shifted toward the use of mild-to-moderate temperatures with encouraging results (10,15,32-35). While conclusive evidence is lacking, these encouraging outcomes coupled with shorter CPB times and avoiding the morbidity of deep hypothermia have led to the increasing clinical acceptance of tepid ACP for arch repair in adults. Moderate hypothermia with ACP has been explored in Europe and Asia for neonatal arch operations, although the typical practice in North America has been to use deep hypothermia with ACP or DHCA alone. Oppido et al. reported 17% early mortality and 8.5% late deaths over a follow-up of up to 50 months in a group of 70 consecutive neonates who underwent the Norwood procedure or aortic arch repair at a nasopharyngeal temperature of 25 °C with ACP (18). Only one patient had postoperative seizures. The authors suggested ACP to be an effective and reliable perfusion strategy that provides a longer safe period for arch repairs and minimizes neurological complications without the need for deep hypothermia. Likewise, Lim et al. (11), Dodge-Khatami et al. (12), Miyaji et al. (20) and Ly et al. (36) demonstrated in neonates and infants the effectiveness of antegrade cerebral perfusion at moderate hypothermia at preserving both cerebral and somatic tissue oxygenation.
Previously, we evaluated moderate (25 °C) and deep (18 °C) hypothermia with ACP in a piglet model for arch operation (37-39). These studies demonstrated improved neuroprotection at 18 and 25 °C with ACP as compared to DHCA alone, with shorter CPB times at 25 °C, and laid the foundation for our clinical practice of moderate hypothermia with ACP during neonatal aortic arch repair. We have employed moderate hypothermia (25 °C) with ACP for all aortic arch reconstructions at the University of Mississippi Medical Center since program inception in April of 2010.
The ideal flow rate for ACP is dependent on many factors and remains to be established. Although the cited literature varies widely in range for ACP from 10 to 100 mL/kg/min, studies utilizing NIRS technology or visual light spectroscopy have indicated that ACP flow rates of greater than 30 mL/kg/min are sufficient to maintain adequate cerebral and somatic oxygen saturations (12,19,40). Admittedly, these findings must be evaluated within the context of temperature and blood gas management (pH versus alpha stat) among other factors. We use ACP at a flow rate of 40–60 mL/kg/min under NIRS guidance to monitor both cerebral and somatic oxygen levels. In the current study, NIRS supports the effectiveness of ACP at 25 °C systemic cooling in maintaining adequate cerebral and lower body perfusion. Although somatic NIRS dropped during ACP, they remained close to baseline levels, suggesting that an ACP flow at 40–60 mL/kg/min was sufficient in maintaining adequate perfusion through collaterals to the lower body and attenuating somatic ischemia during arch operation at 25 °C. This is further supported by favorable postoperative lactate and serum creatinine levels.
Conclusions
The present study suggests that moderate hypothermia (25 °C) with ACP preserves perioperative cerebral oxygenation and serum creatinine in neonates, infants, and children for complex aortic arch operations.
Limitations
The study is limited by the lack of a control group with DHCA alone or ACP at deep hypothermia. Intra-operative electroencephalogram, which does not always correlate with right and left cerebral NIRS, was not performed, and could have disclosed abnormal neurological activity undetected by NIRS. Long-term neurodevelopmental follow-up of these children is required to evaluate the late outcomes of ACP with warmer temperatures and make formal comparison with strategies at 18 °C.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board (2014-0107) approval was obtained for this retrospective study and patient/parent consent was waived.
References
- Barratt-Boyes BG, Nicholls TT, Brandt PW, et al. Aortic arch interruption associated with patent ductus arteriosus, ventricular septal defect, and total anomalous pulmonary venous connection. Total correction in an 8-day-old infant by means of profound hypothermia and limited cardiopulmonary bypass. J Thorac Cardiovasc Surg 1972;63:367-73. [PubMed]
- Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126:1397-403. [Crossref] [PubMed]
- Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 1993;329:1057-64. [Crossref] [PubMed]
- Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation 1998;97:773-9. [Crossref] [PubMed]
- Bellinger DC, Jonas RA, Rappaport LA, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med 1995;332:549-55. [Crossref] [PubMed]
- Bellinger DC, Wypij D, Kuban KC, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 1999;100:526-32. [Crossref] [PubMed]
- Tabbutt S, Nord AS, Jarvik GP, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics 2008;121:476-83. [Crossref] [PubMed]
- Takeda Y, Asou T, Yamamoto N, et al. Arch reconstruction without circulatory arrest in neonates. Asian Cardiovasc Thorac Ann 2005;13:337-40. [Crossref] [PubMed]
- Pigula FA, Gandhi SK, Siewers RD, et al. Regional low-flow perfusion provides somatic circulatory support during neonatal aortic arch surgery. Ann Thorac Surg 2001;72:401-6; discussion 406-7. [Crossref] [PubMed]
- Tsai JY, Pan W, Lemaire SA, et al. Moderate hypothermia during aortic arch surgery is associated with reduced risk of early mortality. J Thorac Cardiovasc Surg 2013;146:662-7. [Crossref] [PubMed]
- Lim HG, Kim WH, Park CS, et al. Usefulness of regional cerebral perfusion combined with coronary perfusion during one-stage total repair of aortic arch anomaly. Ann Thorac Surg 2010;90:50-7. [Crossref] [PubMed]
- Dodge-Khatami J, Gottschalk U, Eulenburg C, et al. Prognostic value of perioperative near-infrared spectroscopy during neonatal and infant congenital heart surgery for adverse in-hospital clinical events. World J Pediatr Congenit Heart Surg 2012;3:221-8. [Crossref] [PubMed]
- Warren DE, Bickler PE, Clark JP, et al. Hypothermia and rewarming injury in hippocampal neurons involve intracellular Ca2+ and glutamate excitotoxicity. Neuroscience 2012;207:316-25. [Crossref] [PubMed]
- Yan TD, Bannon PG, Bavaria J, et al. Consensus on hypothermia in aortic arch surgery. Ann Cardiothorac Surg 2013;2:163-8. [PubMed]
- Zierer A, El-Sayed Ahmad A, et al. Selective antegrade cerebral perfusion and mild (28°C-30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg 2012;144:1042-49. [Crossref] [PubMed]
- Pacini D, Pantaleo A, Di Marco L, et al. Visceral organ protection in aortic arch surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg 2014;46:438-43. [Crossref] [PubMed]
- Rajasinghe HA, Reddy VM, van Son JA, et al. Coarctation repair using end-to-side anastomosis of descending aorta to proximal aortic arch. Ann Thorac Surg 1996;61:840-4. [Crossref] [PubMed]
- Oppido G, Pace Napoleone C, Turci S, et al. Moderately hypothermic cardiopulmonary bypass and low-flow antegrade selective cerebral perfusion for neonatal aortic arch surgery. Ann Thorac Surg 2006;82:2233-9. [Crossref] [PubMed]
- Nasirov T, Mainwaring RD, Reddy VM, et al. Innominate artery cannulation and antegrade cerebral perfusion for aortic arch reconstruction in infants and children. World J Pediatr Congenit Heart Surg 2013;4:356-61. [Crossref] [PubMed]
- Miyaji K, Miyamoto T, Kohira S, et al. Regional high-flow cerebral perfusion improves both cerebral and somatic tissue oxygenation in aortic arch repair. Ann Thorac Surg 2010;90:593-9. [Crossref] [PubMed]
- Fraser CD Jr, Andropoulos DB. Principles of antegrade cerebral perfusion during arch reconstruction in newborns/infants. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2008.61-8. [Crossref] [PubMed]
- Tchervenkov CI, Korkola SJ, Shum-Tim D, et al. Neonatal aortic arch reconstruction avoiding circulatory arrest and direct arch vessel cannulation. Ann Thorac Surg 2001;72:1615-20. [Crossref] [PubMed]
- Kilpack VD, Stayer SA, McKenzie ED, et al. Limiting circulatory arrest using regional low flow perfusion. J Extra Corpor Technol 2004;36:133-8. [PubMed]
- Zhang H, Cheng P, Hou J, et al. Regional cerebral perfusion for surgical correction of neonatal aortic arch obstruction. Perfusion 2009;24:185-9. [Crossref] [PubMed]
- Algra SO, Schouten AN, van Oeveren W, et al. Low-flow antegrade cerebral perfusion attenuates early renal and intestinal injury during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg 2012;144:1323-8, 1328.
- Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 2003;126:1385-96. [Crossref] [PubMed]
- Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 2005;130:1523-30. [Crossref] [PubMed]
- Visconti KJ, Rimmer D, Gauvreau K, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg 2006;82:2207-11; discussion 2211-3. [Crossref] [PubMed]
- Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg 2007;133:880-7. [Crossref] [PubMed]
- Algra SO, Jansen NJ, van der Tweel I, et al. Neurological injury after neonatal cardiac surgery: a randomized, controlled trial of 2 perfusion techniques. Circulation 2014;129:224-33. [Crossref] [PubMed]
- Ohye RG, Goldberg CS, Donohue J, et al. The quest to optimize neurodevelopmental outcomes in neonatal arch reconstruction: the perfusion techniques we use and why we believe in them. J Thorac Cardiovasc Surg 2009;137:803-6. [Crossref] [PubMed]
- Pacini D, Di Marco L, Leone A, et al. Antegrade selective cerebral perfusion and moderate hypothermia in aortic arch surgery: clinical outcomes in elderly patients. Eur J Cardiothorac Surg 2012;42:249-53; discussion 253. [Crossref] [PubMed]
- Leshnower BG, Myung RJ, Chen EP. Aortic arch surgery using moderate hypothermia and unilateral selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:288-95. [PubMed]
- Urbanski PP, Lenos A, Bougioukakis P, et al. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg 2012;41:185-91. [PubMed]
- Tian DH, Wan B, Bannon PG, et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann Cardiothorac Surg 2013;2:148-58. [PubMed]
- Ly M, Roubertie F, Belli E, et al. Continuous cerebral perfusion for aortic arch repair: hypothermia versus normothermia. Ann Thorac Surg 2011;92:942-8; discussion 948. [Crossref] [PubMed]
- Salazar JD, Coleman RD, Griffith S, et al. Selective cerebral perfusion: real-time evidence of brain oxygen and energy metabolism preservation. Ann Thorac Surg 2009;88:162-9. [Crossref] [PubMed]
- Salazar J, Coleman R, Griffith S, et al. Brain preservation with selective cerebral perfusion for operations requiring circulatory arrest: protection at 25 degrees C is similar to 18 degrees C with shorter operating times. Eur J Cardiothorac Surg 2009;36:524-31. [Crossref] [PubMed]
- Allibhai T, DiGeronimo R, Whitin J, et al. Effects of moderate versus deep hypothermic circulatory arrest and selective cerebral perfusion on cerebrospinal fluid proteomic profiles in a piglet model of cardiopulmonary bypass. J Thorac Cardiovasc Surg 2009;138:1290-6. [Crossref] [PubMed]
- Amir G, Ramamoorthy C, Riemer RK, et al. Visual light spectroscopy reflects flow-related changes in brain oxygenation during regional low-flow perfusion and deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2006;132:1307-13. [Crossref] [PubMed]