How to set-up a program of minimally-invasive surgery for congenital heart defects
Introduction
Surgical closure of cardiac defects via a full mid-line sternotomy has been considered the gold standard for over 50 years. The rise of interventional cardiology and new techniques like laparoscopy or thoracoscopy have prompted some groups to explore alternative approaches to median sternotomy (1-7). New adopters and reluctant ones have their own reasons. Added complexity, longer overall and ischemic times and even results account for the balance of the latter.
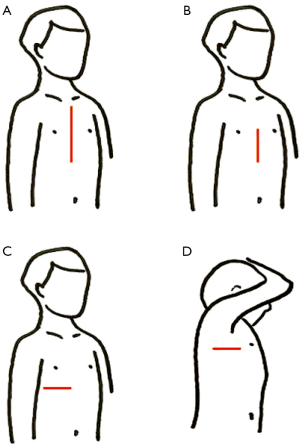
Among the most frequent alternative approaches (Figure 1) we find: lower mini-sternotomy (8-11), right sub-mammary (1,12-16), postero-lateral thoracotomy (17,18) and right axillary incisions (19-23). Main advantages are cosmesis and earlier recovery, as well as saving blood products and lower infection rates. On the other hand, a steep learning curve and technical difficulties in handling some steps (myocardial protection, de-airing maneuvers, and so on) discourage many surgeons to include these minimally invasive procedures within their routine practice.
Trying to schedule a program for starting and teaching minimally invasive pediatric cardiac surgery is a step forward. Few reports can be found in the literature on the topic, if any, except for the right mini-thoracotomy approach employed for mitral repair (23-27) in adult cardiac surgery. In the next paragraphs, we will depict our experience in developing a minimally invasive pediatric cardiac surgery program, pointing out the steps followed as well as the insights provided by the new adopters.
Methods
Upon arrival to a medium-volume centre in which approximately two hundred pump cases per year are carried out, Surgeon A is expected to develop a program of minimally-invasive pediatric cardiac surgery. He has been performing minimally invasive procedures for twelve years in two previous institutions and has produced several papers on the topic (6,16,22,23,28,29), as well as many presentations in local meetings.
The strategy to establish a new program is split in three parts, assuming some overlapping rather than a formal schedule in a three year analysis:
- Performing minimally invasive cases (surgeon A) with every member of the surgical team (surgeons, anesthesiologists, perfusionists, scrub nurses) to let them become familiar and confident with the new approaches;
- Introducing new surgeons to minimally invasive surgery in a stepwise and customized way, according to expertise and skills;
- Developing new strategies together, particularly enhanced by the young staff members.
On the other hand, some quality indicators will be measured, such as:
- Conversion rate. If so, was it to sternotomy or another incision?
- Complications. Trying to figure out whether the alternative approach is to blame for the drawback or if any other cause was responsible for it.
To begin with, a minimally invasive incision will be defined as “surgical approach other than full mid-line sternotomy to perform open heart surgery with extracorporeal circulation”. Three main surgical approaches were introduced by surgeon A: sub-mammary, axillary and lower mini-sternotomy. A single alternative incision gives way either to cannulation maneuvers and correction, with the philosophy of “same steps, same tools, same risks, different approach”. Later in the program (as will be thoroughly displayed in Results and Discussion) several new approaches were added: upper mini-sternotomy, postero-lateral thoracotomy and video-assisted mini-thoracotomy (for which several ports were necessary). Not included in the tables, some off-pump cases via thoracotomy and thoracoscopy were performed, as some experience was acquired by the team.
Before starting any procedure, the proposed incision is drawn with a sterile pen for teaching purposes. Should an enlargement or conversion be needed, security margins are settled (e.g., lower mini-sternotomy enlargement to full sternotomy, or axillary incision conversion to postero-lateral one). Brief description of the minimally invasive approaches:
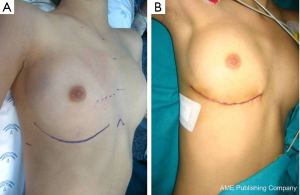
- Sub-mammary. Supine position with the right shoulder slightly elevated and the right arm suspended over the head. Skin incision under the right sub-mammary crease (or 6th intercostal space in children). En-block dissection of subcutaneous tissue and pectoral muscle (30,31). Cage-rib entry in the 4th intercostal space. Full cannulation and correction under cardioplegic arrest (Figures 1C,2);
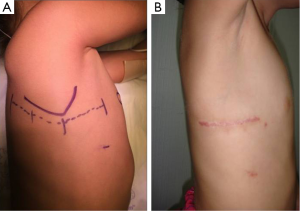
- Axillary. Decubitus lateral position with the right arm suspended over the head. Skin incision in the axillary groove, between anterior and posterior lines. Serratus and latissimus dorsi muscles sparing (28) technique. Cage-rib entry in the 4th intercostal space. Full cannulation and correction under cardioplegic arrest (Figures 1D,3);
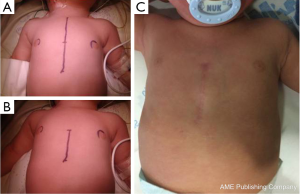
- Lower mini-sternotomy. Supine position. Skin vertical incision below an imaginary line connecting both nipples. Partial lower sternotomy. Regular spreader plus cephalad traction of the sternum. Full cannulation and correction under cardioplegic arrest (Figures 1B,4);
- Upper mini-sternotomy. Supine position. Skin vertical incision above an imaginary line connecting both nipples. Partial upper sternotomy. Full cannulation and correction under cardioplegic arrest;
- Postero-lateral thoracotomy. Decubitus lateral position with the right arm suspended over the head. Skin incision between anterior axillary line and spine (the tip of the scapula being the mid-point). Cage-rib entry in the 4th intercostal space. Full cannulation and correction under cardioplegic arrest (Figure 3A);
- Video-assisted mini-thoracotomy. Supine position with the right shoulder slightly elevated and the right arm secured below the axilla. Mini-skin incision under the right sub-mammary crease. Right jugular and right femoral (arterial and venous) cannulation to institute by-pass. Additional ports for video-assistance, aortic clamp and others. Correction under cardioplegic arrest.
Results
Part one
Surgeon A began his program with sub-mammary, axillary and lower mini-sternotomy cases alternatively, according to age/weight and cardiac condition of every patient. This way, ventricular septal defect (VSD) cases were corrected by mini-sternotomy, atrial septal defect (ASD) patients through an axillary approach, and women with well-defined sub-mammary groove were entered by a sub-mammary incision. The initial three months was time enough to get everyone in the cardiac team comfortable with the changes.
Part two
Surgeons B, C and D were sequentially introduced to lower mini-sternotomy and sub-mammary approaches, according to their own interest and skills. Simple cases (ostium secundum ASD) were selected for this purpose to begin with, followed by VSD closure through lower mini-sternotomy in a customized pattern for every surgeon. By the end of the first year, all surgeons had already performed ASD and VSD cases through lower mini-sternotomy and some ASD closures through a sub-mammary approach.
Surgeon D moved to a different Center in another Country and was substituted by surgeon E, who took up quickly the same method of learning, following the way of surgeons B and C.
On the other hand, Surgeons B and C considered the axillary approach rather cumbersome, and suggested starting a postero-lateral one before attempting the former.
Part three
Surgeon C introduced the upper mini-sternotomy approach for aortic valve surgery with the advice of an adult cardiac surgeon.
As previously stated, the right postero-lateral thoracotomy was suggested by surgeons B and C (and surgeon E, later on) as an initial step before taking up the axillary incision.
Surgeon B suggested moving forward and attempting a thoracoscopic approach. He reviewed the literature (32-37) and contacted a pediatric surgeon with experience in the field from our own Center. After assisting him in thoracoscopic patients (pediatric surgery) and attending a specific course in minimally-invasive thoracoscopy (surgeons B and C), a new program was started.
Surgeon E displayed a sort of algorithm for case-approach, according to age/weight & cardiac defect, resulting in a tailored minimally invasive approach for any given patient.
Table 1 depicts the amount of patients operated on by a minimally invasive approach by every surgeon during the three consecutive years. When compared to the total amount of patients, the ratio of mini-invasive to total pump-cases increased twofold between 2013 and 2015. We have to take into account that 2014 was the first year for Surgeon E, which could explain why the figures are so close between 2013 (20%) and 2014 (22.5%), rather than displaying a steady progression along the three year span.
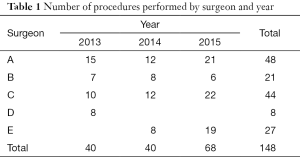
Full table
Increase in percentage of mini-invasive pump cases.
- 2013: 40/201 (20%)
- 2014: 40/178 (22.5%)
- 2015: 68/166 (40%)
Table 2 displays the different approaches by every surgeon. All of us are confident with the lower mini-sternotomy and sub-mammary ones. Only surgeon A is performing the axillary incision up to now, because the remaining staff members feel more comfortable with the postero-lateral approach. The upper mini-sternotomy, introduced by surgeon C, has been taken up by surgeons B and E as well, for aortic valve patients. The video-assisted thoracotomy, led by surgeon B, is applied for ostium secundum ASD patients by surgeons B and C.

Full table
Table 3 shows the distribution of diagnosis and surgeons. Simple conditions, like ASD (ostium secundum, sinus venosus, ostium primum) and VSD have been performed by every surgeon (excepting surgeon D, who left earlier). To sum up, these simple cases account for more than 80% of the whole number of minimally-invasive pump cases. Regarding VSD’s alone, which has been approached by lower mini-sternotomy, the progression has been steady along the three years with a well-defined step up:

Full table
- 2013: 32/40 (80%)—12 VSD
- 2014: 35/40 (87%)—12 VSD
- 2015: 58/68 (82%)—20 VSD
More complex cases (complete atrio-ventricular septal defect, subaortic myectomy (Morrow), scimitar syndrome, tricuspid valve repair) have been performed by surgeon A, expanding the indications of minimally invasive surgery as experience is gained.
Table 4 summarizes the data relating to the approach and cardiac defect, independently of the surgeon. ASD and VSD are the commonest conditions, as expected. Lower mini-sternotomy is the most prevalent approach, given its simplicity (in fact, it is the first alternative incision learned) and the wide range of cardiac defects corrected through this pathway. The sub-mammary incision has been used for any type of ASD and few others; the axillary approach for ostium secundum and sinus venosus ASD, only. At the moment, the upper mini-sternotomy is indicated for aortic valve purposes and the video-assisted thoracotomy for ostium secundum defects.

Full table
Not included in Table 4 which describes pump cases only, some patients were operated on via left thoracotomy without cardio-pulmonary by-pass (one sling left pulmonary artery, two patients with anomalous drainage of left upper pulmonary veins) and video-assisted thoracoscopy [one pericardial window and one left atrial appendage ablation (38) plus clip-exclusion].
Conversion rate
An axillary approach for a sinus venosus ASD had to be converted to a postero-lateral one (just enlarging the skin incision backwards and splitting the latissimus dorsi muscle). Despite the conversion, the postero-lateral approach can still be considered a minimally invasive one. No other conversion was required.
Complications
An ostium primum patient died because progression of diffuse pulmonary vein stenosis three months after repair. A VSD patch-closure developed aortic regurgitation (excessive trimming of redundant tricuspid tissue which happened to be stuck to an aortic cusp) and was re-operated two days later. A valve repair proved unsuccessful and ended up in a Ross-Konno procedure. Two patients (ASD and VSD) required revision for bleeding. The initial approach in all four cases had been via lower mini-sternotomy.
One ASD patient approached via sub-mammary incision developed transient phrenic palsy and continuous pleural effusions. An analysis of the pleural fluid showed lidocaine and, after removal of the trans-thoracic anesthetic line (which was dislodged), both effusion and phrenic palsy resolved. A 55-kg child developed compartment syndrome in the right leg after peripheral cannulation for a video-assisted thoracotomy ASD repair. It was the only case in whom the femoral artery was directly cannulated instead of a graft interposition.
Discussion
Many groups have shifted towards the minimally invasive surgical approaches in pediatrics (1-7). The rationale, beyond cosmesis, is offering the same results with new incisions, when catheter-based interventional procedures are also difficult or contra-indicated. Maybe the future will rely on totally robotic (32) or endoscopic (33-37) surgery, but, for the time being, offering alternative approaches is interesting. Some teams are keen on a single particular approach, whereas others prefer to be familiar with many of them (4-6). Whether this is a strategy or a matter of evolution is beyond the scope of this paper. Currently, the range of incisions different from a full mid-line sternotomy is rich enough to provide us many options. Interestingly, among the literature reviewed, some papers underline the steps to set up programs (24-27). Particularly relevant is the publication by Bonaros et al. (32), in which the authors split every procedure in several parts and analyze them separately, so as to accurately depict anyone´s learning curve. Not only did we need to start a new program, but also to teach and enhance our young staff to develop their own ideas.
The three-step approach to introduce a program of minimally invasive surgery in a new place has proved successful for several reasons. First of all, the results are good and patients/parents are satisfied. Part one (surgeon A introducing the program) allows all members in theatre to get in touch with the novelty, and surgeon A to realize who is enthusiastic and who is reluctant. This way, approaches could be decided according to individual skills and preferences in customized patterns in part two (surgeons B, C, D and E being introduced). Most important was the honest attitude of the staff, not assuming to tackle incisions considered difficult (e.g., axillary one) and suggesting new approaches (part three). As responsible of the team, surgeon A considered not to get involved in the new programs of upper mini-sternotomy for aortic valve cases and video-assisted thoracotomy for ASD patients. The rationale was to let surgeons B and D lead their own projects before incorporating new forthcoming members (E and A): pupils became teachers.
More complex cases were added as experience was gained. Thus, particularly in the last of the three years, the young surgeons were taking up simple cases while surgeon A was performing difficult ones (AVSD, scimitar). As a result, the percentage of minimally invasive cases rose to 40%, doubling the initial rate of 20% during the first year. The lesson is to couple any single patient to a surgeon who is keen either on the defect or on a particular approach, so as to match them in the algorithm of mini-invasive surgery (6,38,39).
Regarding the conversion rate, only one patient had to be switched. The take-home message in a minimally invasive program is trying to convert any patient (when needed) to another minimally invasive approach in an expeditious way. The incision was converted from axillary to postero-lateral incision (again, minimally invasive) by just prolonging posteriorly the already drawn surgical mark and severing the latissimus dorsi muscle. The new program of video-assisted mini-thoracotomy is growing-up under the readiness to convert incisions to a full sub-mammary one, if needed. To date, it has not been necessary to covert a mini-thoracotomy to full mid-line sternotomy.
Before embarking on a minimally invasive program, one has to assume that any drawback is going to be regarded as linked to the alternative approach. Whether it is true or not is irrelevant, unless invasive and minimally-invasive patients are matched. Some of the minor complications we found were definitely related to the approach, like the transient phrenic palsy and the compartment syndrome (40). We have learned how to avoid them (41) in the future.
After gathering some experience, the question is how to move forward with the program? There is no clear answer, since not all surgeons are at the same level of proficiency, or are still in their learning curve. Thinking in terms of contraindications rather than indications, as a last step of training, could be a reasonable marker. In other words, we are not expecting for the “perfect patient” to come and be an ideal candidate for a minimally invasive approach. We rather think about the contraindications, if any, for a minimally invasive procedure in every patient.
The enthusiasm showed by the team members towards new alternative approaches was overwhelming. Not only did the young surgeons take up the new methods quickly (part two), but they quickly suggested new ones to be introduced (part three). To be honest, I had to change my mind from the aphorism “same steps, same tools, same risks, different approach” after the video-assisted mini-thoracotomy program was started. The shift from a different single incision to multi-small approaches one was not in my mind previously, but deserves all credit because it stands for a new paradigm of surgery. The more alternative approaches (5,39) we can offer, the better for the cosmesis of the patients.
Conclusions
Minimally invasive pediatric cardiac surgery is currently becoming a routine practice in many centers worldwide. The different approaches need their own learning curve, either straightforward or a steep one. Our recent experience demonstrates that a comprehensive, three-step schedule allows a safe and custom-made approach to train new surgeons in the field. and enhances enthusiasm in developing further strategies on their own.
A record of conversion-rate and complications should be used as marker of performance and quality standard. The new adopters can take their own training pace according to their level and skills. Interestingly, the wider the offer of approaches, the more ideas come up for new alternative minimally invasive methods. A twofold increase in minimally invasive procedures was observed in two years. The short-medium term results after three years are excellent.
Acknowledgements
The authors would thank the theatre staff for their patience and suggestions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional ethics committee.
References
- Lancaster LL, Mavroudis C, Rees AH, et al. Surgical approach to atrial septal defect in the female. Right thoracotomy versus sternotomy. Am Surg 1990;56:218-21. [PubMed]
- Cremer JT, Böning A, Anssar MB, et al. Different approaches for minimally invasive closure of atrial septal defects. Ann Thorac Surg 1999;67:1648-52. [Crossref] [PubMed]
- Hagl C, Stock U, Haverich A, et al. Evaluation of different minimally invasive techniques in pediatric cardiac surgery: is a full sternotomy always a necessity? Chest 2001;119:622-7. [Crossref] [PubMed]
- del Nido PJ. Minimal incision congenital cardiac surgery. Semin Thorac Cardiovasc Surg 2007;19:319-24. [Crossref] [PubMed]
- Vida VL, Padalino MA, Motta R, et al. Minimally invasive surgical options in pediatric heart surgery. Expert Rev Cardiovasc Ther 2011;9:763-9. [Crossref] [PubMed]
- Gil-Jaurena JM, González-López MT, Pérez-Caballero R, et al. 15 years of minimally invasive paediatric cardiac surgery; development and trends. An Pediatr (Barc) 2016;84:304-10. [Crossref] [PubMed]
- Luo H, Wang J, Qiao C, et al. Evaluation of different minimally invasive techniques in the surgical treatment of atrial septal defect. J Thorac Cardiovasc Surg 2014;148:188-93. [Crossref] [PubMed]
- Bichell DP, Geva T, Bacha EA, et al. Minimal access approach for the repair of atrial septal defect: the initial 135 patients. Ann Thorac Surg 2000;70:115-8. [Crossref] [PubMed]
- Nicholson IA, Bichell DP, Bacha EA, et al. Minimal sternotomy approach for congenital heart operations. Ann Thorac Surg 2001;71:469-72. [Crossref] [PubMed]
- Sun HS, Ma WG, Xu JP, et al. Minimal access heart surgery via lower ministernotomy: experience in 460 cases. Asian Cardiovasc Thorac Ann 2006;14:109-13. [Crossref] [PubMed]
- Garcia Vieites M, Cardenas I, Loyola H, et al. Lower mini-sternotomy in congenital heart disease: just a cosmetic improvement? Interact Cardiovasc Thorac Surg 2015;21:374-8. [Crossref] [PubMed]
- De Mulder W, Vanermen H. Repair of atrial septal defects via limited right anterolateral thoracotomy. Acta Chir Belg 2002;102:450-4. [Crossref] [PubMed]
- Däbritz S, Sachweh J, Walter M, et al. Closure of atrial septal defects via limited right anterolateral thoracotomy as a minimal invasive approach in female patients. Eur J Cardiothorac Surg 1999;15:18-23. [Crossref] [PubMed]
- Mishaly D, Ghosh P, Preisman S. Minimally invasive congenital cardiac surgery through right anterior minithoracotomy approach. Ann Thorac Surg 2008;85:831-5. [Crossref] [PubMed]
- Massetti M, Babatasi G, Rossi A, et al. Operation for atrial septal defect through a right anterolateral thoracotomy: current outcome. Ann Thorac Surg 1996;62:1100-3. [Crossref] [PubMed]
- Gil-Jaurena JM, Murtra M, Gonçalves A, et al. Comparative study of thoracic approaches in atrial septal defect closure. Rev Esp Cardiol 2002;55:1213-6. [Crossref] [PubMed]
- Metras D, Kreitmann B. Correction of cardiac defects through a right thoracotomy in children. J Thorac Cardiovasc Surg 1999;117:1040-2. [Crossref] [PubMed]
- Vida VL, Padalino MA, Bhattarai A, et al. Right posterior-lateral minithoracotomy access for treating congenital heart disease. Ann Thorac Surg 2011;92:2278-80. [Crossref] [PubMed]
- Yang X, Wang D, Wu Q. Repair of atrial septal defect through a minimal right vertical infra-axillary thoracotomy in a beating heart. Ann Thorac Surg 2001;71:2053-4. [Crossref] [PubMed]
- Schreiber C, Bleiziffer S, Kostolny M, et al. Minimally invasive midaxillary muscle sparing thoracotomy for atrial septal defect closure in prepubescent patients. Ann Thorac Surg 2005;80:673-6. [Crossref] [PubMed]
- Prêtre R, Kadner A, Dave H, et al. Right axillary incision: a cosmetically superior approach to repair a wide range of congenital cardiac defects. J Thorac Cardiovasc Surg 2005;130:277-81. [Crossref] [PubMed]
- Gil-Jaurena JM, Zabala J, Conejo L, et al. Minimally invasive pediatric cardiac surgery. Atrial septal defect closure through axillary and submammary approaches. Rev Esp Cardiol 2011;64:208-12. [Crossref] [PubMed]
- Gil-Jaurena JM, Castillo R, Zabala J, et al. Axillary approach for surgical closure of atrial septal defect. An Pediatr (Barc) 2013;79:108-11. [Crossref] [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [Crossref] [PubMed]
- Glower DD, Landolfo KP, Clements F, et al. Mitral valve operation via Port Access versus median sternotomy. Eur J Cardiothorac Surg 1998;14 Suppl 1:S143-7. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003;108 Suppl 1:II48-54. [Crossref] [PubMed]
- Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34:943-52. [Crossref] [PubMed]
- Gil-Jaurena JM, Castillo R, González M. Complete muscle-sparing technique in axillary closure of atrial septal defects. Asian Cardiovasc Thorac Ann 2013;21:756-8. [Crossref] [PubMed]
- Gil-Jaurena JM, Castillo R, Sarria E, et al. Right thoracotomy, off-pump, scimitar syndrome repair in infants. Asian Cardiovasc Thorac Ann 2014;22:353-5. [Crossref] [PubMed]
- Dietl CA, Torres AR, Favaloro RG. Right submammarian thoracotomy in female patients with atrial septal defects and anomalous pulmonary venous connections. Comparison between the transpectoral and subpectoral approaches. J Thorac Cardiovasc Surg 1992;104:723-7. [PubMed]
- Bleiziffer S, Schreiber C, Burgkart R, et al. The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. J Thorac Cardiovasc Surg 2004;127:1474-80. [Crossref] [PubMed]
- Bonaros N, Schachner T, Oehlinger A, et al. Robotically assisted totally endoscopic atrial septal defect repair: insights from operative times, learning curves, and clinical outcome. Ann Thorac Surg 2006;82:687-93. [Crossref] [PubMed]
- Ma ZS, Dong MF, Yin QY, et al. Totally thoracoscopic repair of atrial septal defect without robotic assistance: a single-center experience. J Thorac Cardiovasc Surg 2011;141:1380-3. [Crossref] [PubMed]
- Wang F, Li M, Xu X, et al. Totally thoracoscopic surgical closure of atrial septal defect in small children. Ann Thorac Surg 2011;92:200-3. [Crossref] [PubMed]
- Liu G, Qiao Y, Ma L, et al. Totally thoracoscopic surgery for the treatment of atrial septal defect without of the robotic Da Vinci surgical system. J Cardiothorac Surg 2013;8:119. [Crossref] [PubMed]
- Sabate Rotes A, Burkhart HM, Suri RM, et al. Minimally invasive video-assisted surgical closure of atrial septal defects: a safe approach. World J Pediatr Congenit Heart Surg 2014;5:527-33. [Crossref] [PubMed]
- Zhe Z, Kun H, Xuezeng X, et al. Totally thoracoscopic versus open surgery for closure of atrial septal defect: propensity-score matched comparison. Heart Surg Forum 2014;17:E227-31. [Crossref] [PubMed]
- Pérez-Caballero-Martínez R, Pita-Fernández A, González-López MT, et al. Combined Ablation and Exclusion of the Left Atrial Appendage in a Pediatric Patient: A Minimally Invasive Simplified Approach. Ann Thorac Surg 2016;101:2379-82. [Crossref] [PubMed]
- Vida VL, Tessari C, Fabozzo A, et al. The evolution of the right anterolateral thoracotomy technique for correction of atrial septal defects: cosmetic and functional results in prepubescent patients. Ann Thorac Surg 2013;95:242-7. [Crossref] [PubMed]
- Vida VL, Padalino MA, Boccuzzo G, et al. Near-infrared spectroscopy for monitoring leg perfusion during minimally invasive surgery for patients with congenital heart defects. J Thorac Cardiovasc Surg 2012;143:756-7. [Crossref] [PubMed]
- Vida VL, Padalino MA, Boccuzzo G, et al. Minimally invasive operation for congenital heart disease: a sex-differentiated approach. J Thorac Cardiovasc Surg 2009;138:933-6. [Crossref] [PubMed]


