
Prevalence and risk factors of trimethoprim/sulfamethoxazole-related acute kidney injury in pediatric patients: an observational study from a public database
Introduction
Sulfonamides are a group of antimicrobial drugs with great clinical importance. They are active against gram-positive and gram-negative aerobic bacteria and certain species of fungi and protozoa. They have been widely used in various clinical settings because they are affordable, convenient to use, and have a broad antimicrobial spectrum. Notably, sulfonamides have exhibited their antimicrobial functions in the treatment of infections of multi-drug resistant organisms, such as methicillin-resistant Staphylococcus aureus and carbapenem-resistant organisms (1,2). In China, Trimethoprim/sulfamethoxazole (TMP/SMZ) is the most used sulfonamide. Though well-tolerated, TMP/SMZ can induce adverse events such as hypersensitivity reactions, gastrointestinal upset, and headache (3). Furthermore, its renal toxicity has been reported over years (4-15). A few studies have explored the association between TMP/SMZ use and acute kidney injury (AKI) (12-17). However, most of the existing research focused on adult populations, and the impact of TMP/SMZ use on the development of AKI in pediatric patients is still unclear. In the present study, we investigated the relationship between TMP/SMZ and AKI and identified the potential risk factors of TMP/SMZ-related AKI. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-600/rc).
Methods
Data collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study is a retrospective observational study, using data from a large clinical database named the Paediatric Intensive Care (PIC, version 1.1). PIC is a publicly accessible clinical database with information of more than 12,000 patients admitted to the Children’s Hospital of Zhejiang University (Hangzhou, Zhejiang, China) from 2010 to 2018 (18). Data was extracted using MySQL (version 8.0.16, Oracle Corporation) and Python (version 3.9.1, Python Software Foundation) by 2 independent researchers. The following data of each patient were extracted according to the manager’s guide: demographic characteristics, diagnosis on admission/discharge, dates and results of laboratory tests and prescriptions, and other related data. Baseline laboratory tests were defined as the latest test taken within a week before the treatment started. Concomitant drugs were defined as medications administered for an overlapping duration of more than 48 h with TMP/SMZ treatment, especially angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone antagonists, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid, and other potentially nephrotoxic antimicrobials (e.g., vancomycin).
Patient selection
We screened data on patients treated with TMP/SMZ for at least 48 h. The exclusion criteria were: (I) serum creatinine level >200 µmol/L on admission; (II) patients less than a month old; and (III) patients without data on serum measurements after TMP/SMZ treatment.
Diagnosis of AKI
The pediatric reference change values optimized for AKI in children (pROCK) criterion, which defines AKI as an increase in creatinine levels (≥20 µmol/L) and increase by ≥30% within 7 days, was used for diagnosis (16). TMP/SMZ-associated AKI was defined as AKI which developed between 48 h and 30 days after the start of TMP/SMZ treatment.
Statistical analysis
Data were processed with SPSS for Windows (Version 22.0; SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed using the Student’s t-test or Mann-Whitney U test, whereas categorical variables were analyzed using the chi-squared test or Fisher’s exact test. Variables with P value <0.1 in univariate analysis were analyzed using logistic regression. Statistical significance was set at P<0.05. Missing data were omitted in the analysis.
Results
Patient cohort
Among all the patients in the PIC database, 140 patients received at least one dose of TMP/SMZ. Among them, 27 patients were excluded; 10 were treated with TMP/SMZ for <48 h, 6 did not have serum creatinine measurements after the start of the TMP/SMZ therapy, and 11 had serum creatinine level ≥200 µmol/L on admission. Finally, a total of 113 patients were included for further analysis (Figure 1).
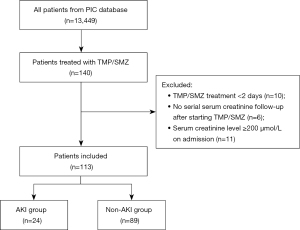
TMP/SMZ use and the development of AKI
In the enrolled cohort, 24 patients (21.2%) met pROCK criteria, while the other 89 constituted the non-AKI group. The baseline characteristics of both groups are described in Table 1. Ninety-two percent (104/113) of the patients were between 3–18 years old and 38.9% (44/113) of the patients were female. Hematological malignancy was the most common underlying systemic condition and were found in 47.8% (54/113) of the patients. Vancomycin was the most used concomitant drug, and a total of 36 patients (31.9%) received TMP/SMZ plus vancomycin treatment.
Table 1
| Characteristics | AKI group (n=24) | Non-AKI group (n=89) | P value | |||
|---|---|---|---|---|---|---|
| N | Result | N | Result | |||
| Age, n (%) | ||||||
| <3 years | 24 | 0 (0) | 89 | 9 (10.1) | 0.104 | |
| 3–18 years | 24 | 24 (100.0) | 89 | 80 (89.9) | 0.104 | |
| Male, n (%) | 24 | 14 (58.3) | 89 | 55 (61.8) | 0.757 | |
| White blood cell count, ×109/L, median (IQR) | 19 | 4.87 (1.49–18.48) | 75 | 3.33 (0.70–8.99) | 0.150 | |
| Platelet count, ×109/L, median (IQR) | 19 | 44.0 (15.5–74.0) | 75 | 94.0 (28.0–248.0) | 0.068 | |
| Alanine transaminase, U/L, median (IQR) | 17 | 18.0 (10.0–31.0) | 68 | 33.0 (19.8–53.5) | 0.035 | |
| Serum lactate, mmol/L, median (IQR) | 19 | 1.50 (1.25–1.75) | 78 | 1.85 (1.40–2.90) | 0.088 | |
| ICU admission, n (%) | 24 | 14 (58.3) | 89 | 50 (56.2) | 0.85 | |
| In-hospital mortality, n (%) | 24 | 7 (29.2) | 89 | 8 (9.0) | 0.010 | |
| Baseline serum creatinine, μmol/L, median (IQR) | 16 | 46.00 (37.00–58.75) | 71 | 37.00 (30.00–47.50) | 0.034 | |
| Duration of TMP/SMZ treatment, days, median (IQR) | 24 | 10.5 (6.0–19.4) | 89 | 7.0 (4.0–12.0) | 0.053 | |
| Concomitant drugs, n (%) | ||||||
| ARB/ACEI | 24 | 2 (8.3) | 89 | 4 (4.5) | 0.457 | |
| NSAIDs | 24 | 7 (29.2) | 89 | 26 (29.2) | 0.996 | |
| Spironolactone | 24 | 2 (8.3) | 89 | 14 (15.7) | 0.356 | |
| Glucocorticoid | 24 | 8 (33.3) | 89 | 15 (16.9) | 0.075 | |
| Vancomycin | 24 | 11 (45.8) | 89 | 25 (28.1) | 0.098 | |
| Piperacillin/tazobactam | 24 | 2 (8.3) | 89 | 10 (11.2) | 0.682 | |
| Meropenem | 24 | 14 (58.3) | 89 | 45 (50.9) | 0.499 | |
| Imipenem/cilastatin | 24 | 7 (29.2) | 89 | 17 (19.1) | 0.285 | |
| Azithromycin | 24 | 3 (12.5) | 89 | 13 (14.6) | 0.793 | |
| Voriconazole | 24 | 11 (45.8) | 89 | 54 (60.7) | 0.192 | |
| Fluconazole | 24 | 0 (0) | 89 | 6 (6.7) | 0.191 | |
| Caspofungin | 24 | 13 (54.2) | 89 | 39 (43.8) | 0.367 | |
| Methotrexate | 24 | 2 (8.3) | 89 | 9 (10.1) | 0.794 | |
| Comorbidities, n (%) | ||||||
| Respiratory tract diseases | 24 | 5 (20.8) | 89 | 18 (20.2) | 0.948 | |
| Heart diseases | 24 | 0 (0) | 89 | 3 (3.4) | 0.362 | |
| Central nervous system diseases | 24 | 0 (0) | 89 | 7 (7.9) | 0.156 | |
| Autoimmune diseases | 24 | 1 (4.2) | 89 | 4 (4.5) | 0.945 | |
| Hematological diseases | 24 | 2 (8.3) | 89 | 9 (10.1) | 0.794 | |
| Digestive system disease | 24 | 0 (0) | 89 | 6 (6.7) | 0.191 | |
| Urinary system disease | 24 | 3 (12.5) | 89 | 5 (5.6) | 0.243 | |
| Hematological malignancy | 24 | 12 (50.0) | 89 | 42 (47.2) | 0.807 | |
| Solid organ malignancy | 24 | 1 (4.2) | 89 | 4 (4.5) | 0.945 | |
AKI, acute kidney injury; IQR, interquartile range; ICU, intensive care unit; TMP/SMZ, trimethoprim/sulfamethoxazole; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; NASAIDs, nonsteroidal anti-inflammatory drugs.
Univariate analysis of patient characteristics for the AKI and non-AKI groups are detailed in Table 1. Baseline serum creatinine level of the AKI group was significantly higher than that of the non-AKI group (46.00 vs. 37.00 µmol/L; P=0.034). A higher in-hospital mortality rate [29.2% (7/24) vs. 9.0% (8/89); P=0.010] was observed in the AKI group than in the non-AKI group. There were no significant differences observed for baseline measurements for white blood cell count, platelet count, and lactate levels between the two groups. Multivariate logistic regression analysis showed that elevated baseline serum creatinine level (OR =1.029; 95% CI: 1.006–1.053; P=0.014) and concomitant use of vancomycin (OR =5.349; 95% CI: 1.381–20.714; P=0.015) are risk factors of developing TMP/SMZ-associated AKI (Table 2).
Table 2
| Parameter | OR | 95% CI | P value |
|---|---|---|---|
| Baseline serum creatinine level | 1.029 | 1.006–1.053 | 0.014 |
| Platelet count | 0.996 | 0.990–1.002 | 0.243 |
| Serum lactate | 0.825 | 0.525–1.295 | 0.403 |
| Concomitant with vancomycin | 5.349 | 1.381–20.714 | 0.015 |
| Concomitant with corticosteroid | 3.049 | 0.709–13.121 | 0.134 |
AKI, acute kidney injury; TMP/SMZ, trimethoprim/sulfamethoxazole; OR, odds ratio; CI, confidence interval.
Survival curves describing baseline serum creatinine and AKI occurrence after TMP/SMZ treatment were drawn (Figure 2). Kaplan-Meier analysis demonstrated significant differences in time of onset of AKI for the patients with different baseline serum creatinine before the use of TMP/SMZ. Baseline serum creatinine ≥40.25 µmol/L was associated with an increased probability of developing AKI (hazard ratio =2.90; P=0.037).
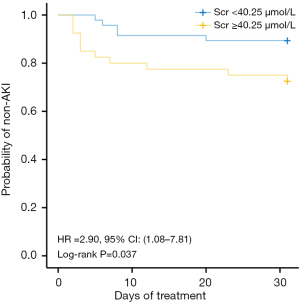
Time of onset of AKI
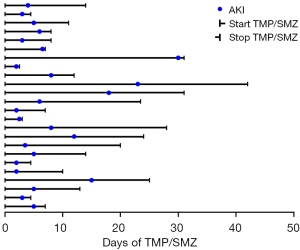
Figure 3 details the timelines of the 24 patients who developed AKI; 19 patients (79.2%) developed AKI within the first 10 days of TMP/SMZ use. The median time of onset of AKI was 4.5 days (interquartile range =3.9).
Discussion
To the best of our knowledge, this is the first study exploring the relationship between the use of TMP/SMZ and the development of AKI in a pediatric population. Our results indicated that 21.2% of the patients that received TMP/SMZ developed AKI, which is higher than the results from previous studies (19,20). Retrospective analysis of 573 patients (12) indicated a TMP/SMZ -associated AKI prevalence of 11.2%. The lower prevalence could be due to the exclusion criteria used. Patients receiving TMP/SMZ for <6 days were excluded; therefore, early onset of AKI that led to treatment withdrawal could have been missed. Another study involving 214 patients reported a TMP/SMZ-associated AKI prevalence of 19.6%, which is similar to our results (20). However, both studies were based on adult populations, and AKI was diagnosed using the Kidney Disease Improving Global Outcomes classification system, which tests for an increase in serum creatinine and serum urea. In our analysis, AKI was diagnosed using pROCK criterion, a newly developed tool for the identification of renal injury in pediatric populations. The results thus might not be comparable, and this may explain the differences in AKI prevalence rates between our study and others. Given the fact that TMP/SMZ is a commonly used antimicrobial in pediatric settings, more studies concerning TMP/SMZ-related AKI prevalence should be carried out.
To explore the risk factors of TMP/SMZ-related AKI in a pediatric population, univariate and multivariate analysis were performed, the results of which showed that a high baseline serum creatinine measurement significantly increased the risk of AKI. Patients with baseline serum creatinine ≥40.25 µmol/L were nearly three times more likely to develop AKI than patients with serum creatinine lower than the cut-off. Similarly, Rajput et al. 2020 reported that low baseline renal function served as a significant predictor for TMP/SMZ-related AKI (20). A pre-evaluation of serum creatinine may thus be essential for predicting the risk of AKI in pediatric patients treated with TMP/SMZ, and kids with baseline creatinine ≥40.25 µmol/L should be monitored.
Notably, we explored the influence of concomitant drugs use on AKI development, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone antagonists, NSAIDs, corticosteroid, vancomycin, and aminoglycosides. Concomitant use of vancomycin with TMP/SMZ significantly increased the risk of AKI. This is quite logical because the main adverse reaction of vancomycin is nephrotoxicity. Previous studies found that in pediatric patients, the incidence of vancomycin-associated AKI was about 20% (17). Ninety percent of vancomycin in its original form is reabsorbed by proximal tubules through glomerular filtration, and it can exert renal toxicity mainly through the induction of oxidative stress response in proximal renal tubule epithelial cells leading to the ischemic necrosis of proximal renal tubules (21). On the other hand, TMP/SMZ’s metabolite which has a low solubility can precipitate in urine, thus, causing hematuria, and in extreme circumstances, interstitial nephritis and tubular necrosis (7-9). The add-up of both mechanisms may further damage the renal tubule and cause deterioration of renal function, which may explain the synergism of vancomycin and TMP/SMZ.
We also analyzed the time of onset of AKI after TMP/SMZ use. According to previous studies (19,20), most AKI incidence occurred within the first 10 days of TMP/SMZ administration, with a medium onset time of 4 days. However, our results did not indicate a significant difference in duration of treatment between the AKI and non-AKI groups [10.5 (6.0–19.4) vs. 7.0 (4.0–12.0) days; P=0.053]. Of note, the relationship between the duration of TMP/SMZ treatment and risk of AKI is unclear. Hence, close monitoring of renal function during the first 10 days of TMP/SMZ use is important.
Notably, this study has several limitations. First, the data on the TMP/SMZ dosage and indication (e.g., prophylaxis or treatment) are not available in PIC database, though other former study suggested that it may be related to the development of AKI (20). Second, the retrospective, single center nature of the study and the relatively small sample size may restrict the reliability of the conclusion. Thus, further prospectively designed studies are required.
Conclusions
This study was a pilot study based on a publicly available database with limited data accessibility. According to our results, elevated baseline serum creatinine levels and concomitant use of vancomycin with TMP/SMZ are associated with an increased probability of AKI in pediatric patients undergoing TMP/SMZ treatment. However, owing to the limitations of the study such as small sample size and lack of data, further studies considering patient baseline conditions together with concomitant prescriptions are required. Moreover, the exact mechanisms of nephrotoxicity in patients need to be investigated. To validate the current findings, a larger prospective cohort study is necessary in future.
Acknowledgments
We would like to thank Prof. Minhao Li, the PIC database manager, for the grant of access to the database as well as its tutorial of use. We also appreciate Dr. Jianda Ge for his technical support to this study. We would like to thank professional editors at Goldediting for their help in polishing our paper.
Funding: This work was supported by the project of Huashan Hospital North (No. HSBY2019007), grant from the Shanghai Municipal Planning Commission of Science and Research Fund (No. 20204Y0441), and funded by the National Natural Science Foundation of China (No. 81573470).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-600/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-600/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-600/coif). MJ reports funding by the project of Huashan Hospital North (No. HSBY2019007) and grant from the Shanghai Municipal Planning Commission of Science and Research Fund (No. 20204Y0441). DL reports funding by the National Natural Science Foundation of China (No. 81573470). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaye KS, Gales AC, Dubourg G. Old antibiotics for multidrug-resistant pathogens: from in vitro activity to clinical outcomes. Int J Antimicrob Agents 2017;49:542-8. [Crossref] [PubMed]
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011;52:285-92. [Crossref] [PubMed]
- Jick H. Adverse reactions to trimethoprim-sulfamethoxazole in hospitalized patients. Rev Infect Dis 1982;4:426-8. [Crossref] [PubMed]
- Kainer G, Rosenberg AR. Effect of co-trimoxazole on the glomerular filtration rate of healthy adults. Chemotherapy 1981;27:229-32. [Crossref] [PubMed]
- Roy MT, First MR, Myre SA, et al. Effect of co-trimoxazole and sulfamethoxazole on serum creatinine in normal subjects. Ther Drug Monit 1982;4:77-9. [Crossref] [PubMed]
- Naderer O, Nafziger AN, Bertino JS Jr. Effects of moderate-dose versus high-dose trimethoprim on serum creatinine and creatinine clearance and adverse reactions. Antimicrob Agents Chemother 1997;41:2466-70. [Crossref] [PubMed]
- Saltissi D, Pusey CD, Rainford DJ. Antibiotic-induced interstitial nephritis? Br Med J 1979;2:50. [Crossref] [PubMed]
- Linton AL, Clark WF, Driedger AA, et al. Acute interstitial nephritis due to drugs: Review of the literature with a report of nine cases. Ann Intern Med 1980;93:735-41. [Crossref] [PubMed]
- Smith EJ, Light JA, Filo RS, et al. Interstitial nephritis caused by trimethoprim-sulfamethoxazole in renal transplant recipients. JAMA 1980;244:360-1. [Crossref] [PubMed]
- Alappan R, Perazella MA, Buller GK. Hyperkalemia in hospitalized patients treated with trimethoprim-sulfamethoxazole. Ann Intern Med 1996;124:316-20. [Crossref] [PubMed]
- Mori H, Kuroda Y, Imamura S, et al. Hyponatremia and/or hyperkalemia in patients treated with the standard dose of trimethoprim-sulfamethoxazole. Intern Med 2003;42:665-9. [Crossref] [PubMed]
- Berglund F, Killander J, Pompeius R. Effect of trimethoprim-sulfamethoxazole on the renal excretion of creatinine in man. J Urol 1975;114:802-8. [Crossref] [PubMed]
- Shouval D, Ligumsky M, Ben-Ishay D. Effect of co-trimoxazole on normal creatinine clearance. Lancet 1978;1:244-5. [Crossref] [PubMed]
- Trollfors B, Wahl M, Alestig K. Co-trimoxazole, creatinine and renal function. J Infect 1980;2:221-6. [Crossref] [PubMed]
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ 2011;343:d5228. [Crossref] [PubMed]
- Xu X, Nie S, Zhang A, et al. A New Criterion for Pediatric AKI Based on the Reference Change Value of Serum Creatinine. J Am Soc Nephrol 2018;29:2432-42. [Crossref] [PubMed]
- Fiorito TM, Luther MK, Dennehy PH, et al. Nephrotoxicity With Vancomycin in the Pediatric Population: A Systematic Review and Meta-Analysis. Pediatr Infect Dis J 2018;37:654-61. [Crossref] [PubMed]
- Zeng X, Yu G, Lu Y, et al. PIC, a paediatric-specific intensive care database. Sci Data 2020;7:14. [Crossref] [PubMed]
- Fraser TN, Avellaneda AA, Graviss EA, et al. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J Antimicrob Chemother 2012;67:1271-7. [Crossref] [PubMed]
- Rajput J, Moore LSP, Mughal N, et al. Evaluating the risk of hyperkalaemia and acute kidney injury with cotrimoxazole: a retrospective observational study. Clin Microbiol Infect 2020;26:1651-7. [Crossref] [PubMed]
- Jeffres MN. The Whole Price of Vancomycin: Toxicities, Troughs, and Time. Drugs 2017;77:1143-54. [Crossref] [PubMed]


