
Establishment of a predictive model for purulent meningitis in preterm infants
Introduction
Purulent meningitis (PM) is a common infectious disease in the neonatal period that may cause long-term neurological sequelae. The incidence of PM is significantly higher in the neonatal period than in other periods. Incidence of PM was 0.1–0.4 per 1,000 live births in neonates, and 10–15-fold higher in very low-birth weight (VLBW) infants reaching 2/1,000 (1). Even in developed countries, mortality from neonatal meningitis was 5–20% and neurological sequelae was 20–50% (2). Neonates are at higher risk of meningitis because of immaturity in humoral and cellular immunity Premature infants are at higher risk of PM due to immaturity in humoral and cellular immunity and blood–brain barrier development. Neonatal PM lacks specific symptoms and may cause an abnormal body temperature, irritability, drowsiness, apnea, and convulsions. These symptoms in preterm infants are more atypical. It is difficult to differentiate PM from neonatal sepsis, bilirubin encephalopathy, and intracranial hemorrhage based on clinical symptoms alone. And no haematological test can distinguish PM from sepsis and other diseases.
PM is mainly diagnosed based on cerebrospinal fluid (CSF) test, and CSF specimens are usually obtained through a lumbar puncture (3). However, a lumbar puncture is an invasive and difficult operation that is prone to causing local injury or to puncture failure, and its success is highly dependent on the experience of the surgeon. Unsuccess rates of lumbar puncture procedures reported ranging from 22.5% to 42.9% in neonates (4-6). Preterm is often accompanied by poor conditions, which could increase the difficulty and risk of puncture (7).
It has been estimated that 15–30% of infants with CSF proven meningitis have negative blood cultures and about 15% of neonates with negative blood cultures have positive CSF cultures (1). At present, it is controversial that patients need lumbar puncture to diagnosis or exclude PM. Many centers perform lumbar puncture for neonate with clinical symptoms, or with central nervous system symptoms such as apnea and convulsions, or with positive blood cultures. A previous multicenter study showed that a sequential algorithm based on five predictive factors, including clinical manifestations and laboratory results, could be used to screen for low-risk meningitis in full-term infants, negating the need to conduct invasive lumbar punctures in newborns (8). But there were no similar studies on PM in premature infants. Many factors are associated with the occurrence of preterm PM. Organisms present in the mother can be passed to the fetus by blood, rectovaginal, amniotic fluid and placenta, causing neonate PM (9). Some hematology tests, such as white blood cell (WBC), C-reactive protein (CRP), and procalcitonin (PCT) tests, are of limited value individually due to their low sensitivity and cannot be used to predict PM (10).
The aim of the current study was to establish a convenient and effective clinical predictive model on the base of perinatal factors to assess the risk of PM, in hopes of helping clinicians develop new diagnostic and treatment strategies for preterm. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-236/rc).
Methods
Data Collection and Diagnosis
Premature infants who were admitted to The First Affiliated Hospital of Zhengzhou University from September 2017 to March 2020 were enrolled in this study. All the patients underwent lumbar puncture. CSF culture is the gold standard for diagnosing meningitis. Based on prior studies (11,12), the PM was diagnosed if met any criterion of the following: (I) positive CSF culture; or (II) CSF pleocytosis (WBC ≥20/mm3) with a predominance of polymorphonucleocytes (>50%), and CSF glucose <2.2 mmol/L or CSF glucose 40% lower than normal blood glucose level or CSF protein is greater than 1880 mg/L. If the lumbar puncture generated hemorrhagic CSF, the CSF WBC count was calculated based on the ratio of the WBCs to neutrophils on the same day. Infants were excluded from the study if they met any of the following exclusion criteria: (I) full-term infants with gestational age (GA) ≥37 weeks; (II) congenital neurological developmental malformations, genetic metabolic diseases, chromosomal abnormalities, or had died; (III) their family members abandoned the treatment or refused to allow the lumbar puncture to be performed during the hospitalization; and/or (IV) incomplete general information.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University (ethics No. KY-2020-0514). As it was a retrospective study, informed consent was not required.
Risk factors
Based on clinical experience and prior report (3), we collected data encompassing maternal diseases and neonatal clinical features. The maternal factors contain: maternal age, history of abnormal pregnancy, antenatal corticosteroids, delivery method, anesthesia during delivery, embryo transfer, gestational hypertension, gestational diabetes, liver injury during pregnancy, intrahepatic cholestasis of pregnancy (ICP), premature rupture of membranes, maternal WBC count and classification, and platelet count before delivery, single birth/multiple births, abnormalities of placental, umbilical and amniotic fluid. Neonatal factors include sex, GA, birth weight (BW), 1- and 5-minute Apgar scores, vital signs at admission (including temperature, heart rate, respiration, and blood pressure), partial pressure of oxygen, partial pressure of carbon dioxide, bases excess value (BE), blood glucose, hematological indicators within 24 hours after birth (including hemoglobin, urea nitrogen, creatinine, albumin, creatine kinase isoenzyme, C-reactive protein, PCT, and N-terminal brain natriuretic peptide (NT-proBNP)), mechanical ventilation, non-invasive biphasic positive airway pressure (BiPAP), continuous positive airway pressure (CPAP).
Statistical analysis
All statistical analyses were performed using R 3.63 (https://www.r-project.org/). The “glmnet” package in R software was used to perform the least absolute shrinkage and selection operator (LASSO) regression to screen the best predictors of PM. The “rms” package was used to incorporate the factors selected by LASSO regression into the multivariate logistic regression analysis to build a prediction model. Differences with P<0.05 were considered statistically significant.
Establishment of the Predictive Model and Nomogram
Based on the collection data from premature infants, we use LASSO regression analysis to identify optimal risk factors affecting PM. The minimum turning parameter λ was determined with cross-validation. The factors screened by LASSO regression were used to establish the prediction model according to logistic regression analysis. Then we present the prediction model as a nomogram. A decision curve analysis was conducted to evaluate the clinical utility of the nomogram.
The Brier score, calibration slope, and concordance (C)-index were used to evaluate the performance of the model. The Brier score closed to 0, the standard slope closed to 1, and the C-index closed to 1, indicating good predictive ability. In addition to these numeric measures, we used the calibration plot and receiver operating characteristic curve to display the calibration and discrimination aspects of our final model.
Results
Baseline clinical characteristics
A total of 168 infants were enrolled in this study, 80 boys and 88 girls. The GA was 26.43–36.86 weeks (32.45±2.79 weeks), and the BW was 700–3,400 g (1,814.05±568.84 g). The blood culture of 12 preterms were positive, 4 cases with Staphylococcus, 4 cases with Klebsiella pneumoniae, 2 cases with Enterococcus faecalis, 1 case with Listeria, and 1 case with Streptococcus salivarius. The CSF culture of 3 preterms were positive, 1 case with Staphylococcus, 1 case with Klebsiella pneumoniae, and 1 case with Listeria. Among the preterm infants, 77 with PM and 91 without PM. The general information of the included children is set out in Table 1.
Table 1
| Variable | Overall cohort |
|---|---|
| Sex (male/female), (%) | 80 (47.6)/88 (52.4) |
| Gestational age (Weeks) | 32.45±2.79 |
| Birth weight (g) | 1,814.05±568.84 |
| Single birth/multiple birth | 129 (76.8)/39 (23.2) |
| 1-min Apgar | 7.77±2.1 |
| 5-min Apgar | 8.94±1.47 |
| Temperature (℃) | 35.95±0.51 |
| Heart rate (beats/min) | 132.48±16.81 |
| Respiratory rate (times/min) | 41.08±10.46 |
| Systolic blood pressure (mmHg) | 62.39±10.42 |
| Diastolic blood pressure (mmHg) | 34.61±8.56 |
| Partial pressure of oxygen (mmHg) | 63.91±23.48 |
| Partial pressure of carbon dioxide (mmHg) | 49.38±12.98 |
| Bases excess (mmol/L) | −4.34±3.98 |
| Blood glucose (mmol/L) | 3.78±1.91 |
| Hemoglobin (g/L) | 160.58±24.03 |
| Blood urea nitrogen (mmol/L) | 4.10±2.46 |
| Creatinine (µmol/L) | 59.98±23.21 |
| Albumin (g/L) | 30.29±5.16 |
| Creatine kinase Isoenzyme (U/L) | 216.98±278.80 |
| C-reactive protein (mg/L, postnatal day 1) | 6.10±9.68 |
| Procalcitonin (ng/mL, postnatal day 1) | 4.40±7.28 |
| NT-proBNP (ng/L, postnatal day 1) | 6,017.84±8,460.27 |
| Ventilation (days) | 2.54±6.47 |
| BiPAP (days) | 1.30±3.78 |
| CPAP (days) | 5.98±8.30 |
| Mothers’ information | |
| Placenta anomalies | 55 (32.7%) |
| Umbilical cord anomalies | 49 (29.2%) |
| Amniotic fluid anomalies | 28 (16.7%) |
| Premature rupture of membranes | 57 (33.9%) |
| History of abnormal pregnancy | 16 (9.5%) |
| Antenatal corticosteroids | 38 (22.6%) |
| Cesarean delivery/natural labor, (%) | 127 (75.6)/41 (24.4) |
| General anesthesia | 47 (28.0%) |
| Embryo transplantation | 35 (20.8%) |
| Gestational hypertension | 42 (25.0%) |
| Gestational diabetes mellitus | 29 (17.3%) |
| Abnormal liver function in pregnancy | 2 (1.2%) |
| Intrahepatic cholestasis of pregnancy | 1 (0.6%) |
| Age (years) | 30.84±5.57 |
| White blood cells (×109/L) | 9.89±3.11 |
| Neutrophil granulocytes (×109/L) | 8.05±5.71 |
| Lymphocytes (×109/L) | 1.72±1.67 |
| Blood platelet count (×109/L) | 190.70±62.51 |
NT-proBNP, N-terminal brain natriuretic peptide; BiPAP, biphasic positive airway pressure; CPAP, continuous positive airway pressure.
Selection of perinatal characteristics
LASSO regression was used to screen the independent risk factors for PM (Figure 1A,1B). The coefficient, λ, decreases a greater number of variables. When λ is optimal, the coefficient of the excluded variables is compressed to 0, while the coefficients of the variables left in the model were nonzero. The results of our analysis indicated that the optimal value of λ was 0.080960, and the logarithm was (λ) = −2.5138. According to LASSO analysis results, the 43 perinatal factors were reduced to 7 potential predictors. The influencing factors include PCT on the 1st day after birth, prenatal glucocorticoid use, albumin, the 1-minute Apgar score, the duration of BiPAP, hemoglobin, and sex (Table 2).
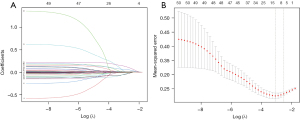
Table 2
| Variable | β | P value |
|---|---|---|
| PCT 1st | 0.1258 | 0.0006 |
| Glucocorticoid | 1.2542 | 0.0096 |
| Albumin | –0.1089 | 0.0066 |
| 1-min Apgar | –0.2557 | 0.0118 |
| BiPAP | –0.1838 | 0.0195 |
| Hb | –0.0172 | 0.0290 |
| Sex | –0.8131 | 0.0354 |
PM, purulent meningitis; PCT 1st, procalcitonin on the 1st day after birth; BiPAP, biphasic positive airway pressure; Hb, hemoglobin.
Development of nomogram
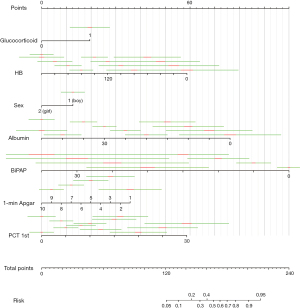
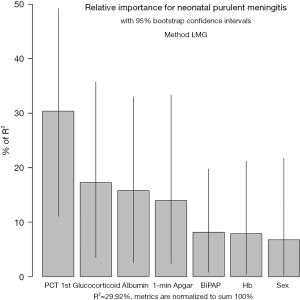
On the basis of the logistic regression results, 7 variables with P value <0.05 were used to construct the risk prediction nomogram (Figure 2). The C-index of the nomogram was 0.82018 (95% confidence interval: 0.75463–0.88573), indicating that the model had good discriminant and predictive abilities. Additionally, we analyzed the degree of variance explained by each influencing factor for PM in the preterm infants, and the results showed that PCT on the 1st day after birth, prenatal glucocorticoid use, albumin, and the 1-minute Apgar score contributed the most to PM (Figure 3).
Verification of accuracy for the prediction model
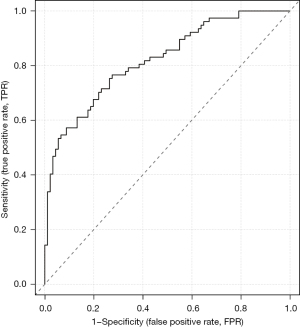
The accuracy of the prediction model was verified. The ROC of the nomogram showed that indicated that the model had relatively high accuracy (Figure 4). Additionally, a calibration curve (Figure 5), decision curve (Figure 6), and clinical impact curve (Figure 7) were plotted to evaluate the prediction model. The Brier score was 0.17, the calibration slope was 0.966, and the C-index was 0.82 (95% CI: 0.75–0.89).
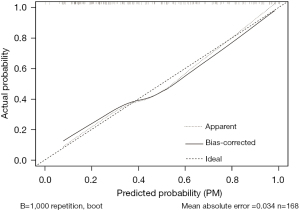
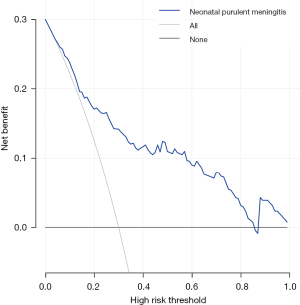
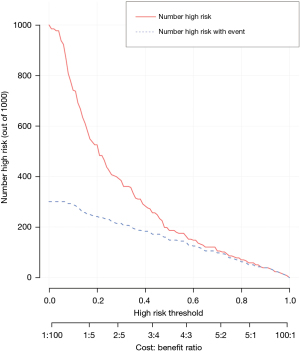
Discussion
This study established a prediction model for PM in preterm infants. We found that PCT on the 1st day after birth, prenatal glucocorticoid use, albumin, the 1-minute Apgar score, the duration of BiPAP and CPAP, hemoglobin, and sex were influencing factors. The nomogram comprising these clinical variables predicted the risk of PM in preterm infants well. The use of the prediction model can help clinicians decide whether to perform lumbar puncture and antibiotic use in preterm infants with high-risk factors, in turn reduce missing meningitis diagnosis and the occurrence of sequelae, also reduce unnecessary operations and treatments.
Our prediction model showed that PCT levels and prenatal glucocorticoids on the 1st day after birth are positively correlated with PM in preterm infants. Conversely, the serum albumin level, the Apgar score at 1 minute after birth, the duration of non-invasive BiPAP and CPAP, and hemoglobin level 1 day after birth are negatively correlated with PM in preterm infants. In addition, the occurrence of PM in preterm infants is also correlated with sex.
Among the factors affecting PM in preterm infants, the PCT level on the 1st day after birth had the greatest effect. PCT is a pre-peptide of calcitonin derived from the calcitonin gene-related peptide I located on chromosome 11. PCT can be detected within 2 hours of infection and peaks at approximately 12–24 hours (13). PCT can be used to diagnose infectious diseases at an early stage and predict the severity of infection and has a sensitivity and specificity of 83–100% and 70–100%, respectively (14). A physiological increase in PCT occurs in newborns 24–48 hours after birth and is negatively correlated with GA and BW. A 100-g increase in BW has been shown to be associated with a 2.2% decrease in PCT, and a 1-week increase in GA has been shown to be associated with an 11.4% decrease in PCT (15). A previous study in children <2 months of age shown that serum PCT combined with routine urine analysis and neutrophil count results can be used to identify febrile infants at risk of severe bacterial infection, which reduces the need to use lumbar punctures to exclude PM (16). This study found that the higher the PCT level on the 1st day after birth, the higher the risk of PM in preterm infants. The mean PCT in the PM group and non-PM group were 7.12±0.98 ng/mL and 2.09±0.52 ng/mL, respectively. PCT needs to be dynamically assessed based on GA, BW, and age. If PCT is significantly higher than the upper limit of the normal range and other factors in the prediction model are abnormal, then it is necessary to actively evaluate whether a lumbar puncture is necessary.
The prenatal use of glucocorticoids is one of the most important methods for preventing respiratory distress syndrome (RDS) in preterm infants. It can reduce the incidence and mortality of neonatal RDS, intraventricular hemorrhage (IVH), and necrotizing enterocolitis, and has been widely used in clinical practice. The effects of the prenatal use of exogenous glucocorticoids by mothers on the immune system of fetuses during the developmental process is still unclear. However, a few studies have shown that glucocorticoid exposure can change the long-term physiological and cellular responses of offspring to infection and inflammation (17,18). A clinical study shown that after prenatal glucocorticoid treatment, the number of lymphocytes in preterm infants decreases and the total number of WBC and neutrophils increases (19). Additionally, animal experiments have shown that treating sows with cortisol during pregnancy increases the febrile response of female offspring to lipopolysaccharide stimulation (20). Roberts et al. showed that the routine use of glucocorticoids promotes fetal lung maturity in women with premature rupture of membranes and at risk of preterm birth, GA <34 weeks, and did not increase the risk of maternal and neonatal infections (21). However, glucocorticoids may lead to fetal growth restriction (22) and increase the risk of metabolic diseases, such as elevated blood pressure and increased insulin resistance (23,24). The use of multiple courses of prenatal glucocorticoids increases the risk of neonatal sepsis and mortality (25,26). In some pregnant women with special conditions, such as twin pregnancies and intrauterine growth retardation (27,28), the application of glucocorticoids will not promote fetal lung maturation. The results of our study showed that the prenatal use of glucocorticoids increased the risk of PM in preterm infants; however, the specific mechanism needs to be further studied.
The prediction model showed that a low Apgar score at 1 minute after birth, hypoalbuminemia, anemia, and the duration of BiPAP and CPAP were associated with PM in preterm infants. The Apgar score is a method for rapidly assessing the general status of newborns after birth. Many factors can lead to low Apgar scores after birth, including intrauterine hypoxia, delivery under general anesthesia, and congenital developmental malformations. The albumin level in children with sepsis is significantly reduced and further decreases with disease aggravation (29). Anemia is a common symptom of severe infection and is associated with iatrogenic blood loss and serum iron content (30). After birth, preterm infants often need BiPAP and CPAP to improve respiration for various reasons. The more severe the disease, the longer use duration of assisted ventilation (31). Compared to female preterm infants, male preterm infants are more likely to develop PM (32); however, the specific mechanism needs to be further studied.
A study showed that the premature rupture of membranes is also a high-risk factor for PM in preterm infants (33). However, preterm infants with prematurely ruptured membranes have increased PCT after birth. This study had a multicollinearity problem, such that variables with multicollinearity were excluded by the LASSO regression; thus, this factor was not included in the model developed in this study.
This study had several limitations. First, the sample size was relatively small; a larger sample size would have had better predictive value. Second, while an internal verification was conducted, no external verification was conducted, which limits the large-scale generalization and application of this model.
Conclusions
Our prediction model could predict the risk of PM in preterm infants. Using this prediction model, it may be able to provide reference to determine whether lumbar puncture is performed and whether antibiotics are applied as soon as possible.
Acknowledgments
Funding: This study was supported by Henan Province Medical Science and Technology Research Plan (Provincial-Ministerial Joint Project, No. SBGJ2018040).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-236/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-236/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-236/coif). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University (ethics No. KY-2020-0514). As it was a retrospective study, informed consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baud O, Aujard Y. Neonatal bacterial meningitis. Handb Clin Neurol 2013;112:1109-13. [Crossref] [PubMed]
- Kohli-Lynch M, Russell NJ, Seale AC, et al. Neurodevelopmental Impairment in Children After Group B Streptococcal Disease Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis 2017;65:S190-9. [Crossref] [PubMed]
- Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol 2015;42:29-45. vii-viii. [Crossref] [PubMed]
- Glatstein MM, Zucker-Toledano M, Arik A, et al. Incidence of traumatic lumbar puncture: experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila) 2011;50:1005-9. [Crossref] [PubMed]
- Greenberg RG, Smith PB, Cotten CM, et al. Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count. Pediatr Infect Dis J 2008;27:1047-51. [Crossref] [PubMed]
- Sievänen H, Palmu S, Kari J, et al. Incidence of Traumatic Lumbar Punctures in Neonates and Infants. Am J Perinatol 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Bedetti L, Lugli L, Marrozzini L, et al. Safety and Success of Lumbar Puncture in Young Infants: A Prospective Observational Study. Front Pediatr 2021;9:692652. [Crossref] [PubMed]
- Chen Y, Yin Z, Gong X, et al. A sequential guide to identify neonates with low bacterial meningitis risk: a multicenter study. Ann Clin Transl Neurol 2021;8:1132-40. [Crossref] [PubMed]
- Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol 2015;42:29-viii. [Crossref] [PubMed]
- Chang SSY, Lim AZ, Ong GY, et al. Predictors of serious bacterial infections using serum biomarkers in an infant population aged 0 to 90 days: a prospective cohort study. BMJ Paediatr Open 2021;5:e000861. [Crossref] [PubMed]
- Hernández Ortiz OH, García García HI, Muñoz Ramírez F, et al. Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg 2018;128:262-71. [Crossref] [PubMed]
- Tan J, Kan J, Qiu G, et al. Clinical Prognosis in Neonatal Bacterial Meningitis: The Role of Cerebrospinal Fluid Protein. PLoS One 2015;10:e0141620. [Crossref] [PubMed]
- Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994;79:1605-8. [PubMed]
- van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis 2004;4:620-30. [Crossref] [PubMed]
- Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta 2011;412:1053-9. [Crossref] [PubMed]
- Kuppermann N, Dayan PS, Levine DA, et al. A Clinical Prediction Rule to Identify Febrile Infants 60 Days and Younger at Low Risk for Serious Bacterial Infections. JAMA Pediatr 2019;173:342-51. [Crossref] [PubMed]
- Alexander N, Rosenlöcher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab 2012;97:3538-44. [Crossref] [PubMed]
- Pole JD, Mustard CA, To T, et al. Antenatal steroid therapy for fetal lung maturation and the subsequent risk of childhood asthma: a longitudinal analysis. J Pregnancy 2010;2010:789748. [Crossref] [PubMed]
- Kadanali S, Ingeç M, Küçüközkan T, et al. Changes in leukocyte, granulocyte and lymphocyte counts following antenatal betamethasone administration to pregnant women. Int J Gynaecol Obstet 1997;58:269-74. [Crossref] [PubMed]
- de Groot J, Kranendonk G, Fillerup M, et al. Response to LPS in female offspring from sows treated with cortisol during pregnancy. Physiol Behav 2007;90:612-8. [Crossref] [PubMed]
- Roberts D, Brown J, Medley N, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [PubMed]
- Braun T, Sloboda DM, Tutschek B, et al. Fetal and neonatal outcomes after term and preterm delivery following betamethasone administration. Int J Gynaecol Obstet 2015;130:64-9. [Crossref] [PubMed]
- Doyle LW, Ford GW, Davis NM, et al. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137-42. [Crossref] [PubMed]
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005;365:1856-62. [Crossref] [PubMed]
- Vermillion ST, Soper DE, Chasedunn-Roark J. Neonatal sepsis after betamethasone administration to patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1999;181:320-7. [Crossref] [PubMed]
- Vermillion ST, Soper DE, Newman RB. Neonatal sepsis and death after multiple courses of antenatal betamethasone therapy. Am J Obstet Gynecol 2000;183:810-4. [Crossref] [PubMed]
- Ushida T, Kotani T, Sadachi R, et al. Antenatal Corticosteroids and Outcomes in Preterm Twins. Obstet Gynecol 2020;135:1387-97. [Crossref] [PubMed]
- Blankenship SA, Brown KE, Simon LE, et al. Antenatal corticosteroids in preterm small-for-gestational age infants: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100215. [Crossref] [PubMed]
- Fan JH, Zhu YM, Zhang XP. Correlation of hypoproteinemia with C-reactive protein and procalcitonin in children with sepsis. Zhongguo Dang Dai Er Ke Za Zhi 2010;12:870-3. [PubMed]
- Hayden SJ, Albert TJ, Watkins TR, et al. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med 2012;185:1049-57. [Crossref] [PubMed]
- Wilson A, Gardner MN, Armstrong MA, et al. Neonatal assisted ventilation: predictors, frequency, and duration in a mature managed care organization. Pediatrics 2000;105:822-30. [Crossref] [PubMed]
- Haddad-Boubaker S, Lakhal M, Fathallah C, et al. Epidemiological study of bacterial meningitis in Tunisian children, beyond neonatal age, using molecular methods: 2014-2017. Afr Health Sci 2020;20:1124-32. [Crossref] [PubMed]
- Wu J, Liu J, Feng ZC, et al. Influence of premature rupture of membranes on neonatal health. Zhonghua Er Ke Za Zhi 2009;47:452-6. [PubMed]


