
Suppressing the activity of CXCR4 down-regulates the expression of renal fibrosis related genes in primary glomerular cells
Introduction
Renal fibrosis is defined as excessive growth, sclerosis, or scarring of kidney tissue due to excessive accumulation of extracellular matrix components, such as collagens. Severe cases of renal fibrosis may result in renal dysfunction (1). Renal fibrosis can be induced by almost all kinds of chronic renal diseases or injuries, such as sustained infections, autoimmune reactions, anaphylaxis, chemical injuries, radiation, and mechanical injuries (2). Chemokines are peptides with molecular weight ranging from 8 to 12 kDa, and are named for its chemotaxis against leukocytes. Chemokines have been reported to be involved in the development and progression of fibrosis by recruiting myofibroblasts, macrophages, and other effector cells to the site of injury (3,4). It has been demonstrated that pulmonary fibrosis can be significantly ameliorated by downregulating the expression chemokines, such as C-C motif chemokine ligand (CCL)2 and CCL3, or by blocking their receptors (5-7). In addition, the expression of fibrosis inducers, such as interleukin (IL)-4 and IL-13, could be suppressed by blocking the down-stream signaling pathways of CCL3/CCL2 (8,9). These reports verified the significance of chemokines in fibrosis. The C-X-C chemokine receptor (CXCR)4 is the receptor of CXCL12 (SDF1) and the main function of CXCL12/CXCR4 is to regulate the transport of hematopoietic cells and secondary lymphoid tissue structures (10), which play an important role in the growth and development of renal vessels (11). A study has shown that CXCR4 is a potential target for preventing renal fibrosis in patients with nephrolithiasis (12). Under renal ischemia, the expression of CXCR4 is elevated (13). Recently, CXCL12/CXCR4 was shown to promote the progression of renal fibrosis in unilateral ureteral ligated mice and rats with chronic transplanted kidney disease (14,15). However, the impact of CXCR4 on renal fibrosis has only been reported in animal experiments, which lacked fundamental investigations on a cellular level. Moreover, renal fibrosis is a common pathophysiological change after the end-stage of chronic kidney disease. Inflammation also plays an important role in renal fibrosis, but non-inflammatory factors (such as ischemia and hypoxia) may also lead to renal fibrosis in the course of renal fibrosis. Therefore, we explore the effect of CXCR4 gene on renal fibrosis from the perspective of non-inflammation. Apart from blood cells and immune cells, the glomeruli consists of glomerular basement membrane, mesangial matrix, glomerular endothelial cells, mesangial cells, and podocytes. Podocytes are targets of inflammatory and non-inflammatory injury in glomerular diseases, when podocytes are stimulated by various pathologies, it leads to the fusion and disappearance of podocytes and the abnormal number, shape and density of podocytes. The changes of pore membrane and cytoskeleton proteins destroy the integrity of glomerular filtration barrier and function, and cause mesangial cell proliferation and extracellular matrix metabolism disorder, resulting in glomerulosclerosis. The present study suppressed the activity of CXCR4 in the glomerular endothelial cells, mesangial cells, and podocytes by transfecting a short hairpin (sh)RNA or utilizing the CXCR4 inhibitor, to explore the impacts of CXCR4 suppression on the expression of fibrosis-related genes. This investigation provided novel insights into the regulatory properties of CXCR4 in glomerular cells under a non-inflammatory state. We present the following article in accordance with the MDAR and ARRIVE reporting checklists (available at https://tp.amegroups.com/article/view/10.21037/tp-22-157/rc).
Methods
Isolation and culture of primary glomerular endothelial cells, mesangial cells, and podocytes
A protocol was prepared before the study without registration. Animal experiments were performed under a project license (No. 2020052701) granted by ethics committee of Jiangxi Provincial Children’s Hospital, in compliance with Jiangxi Provincial Children’s Hospital institutional guidelines for the care and use of animals.
Mouse were sacrificed and the kidneys were isolated and placed into Hank’s medium containing penicillin and streptomycin. Mouse purchased from Hunan slake Jingda experimental animal Co., Ltd. [License No: SCXK(Xiang)2021-0002], age 26-30 days. The kidney capsule was removed and the renal cortex was clipped into pieces. The glomerular endothelial cells were digested using 0.1% collagenase II solution, and the mesangial cells and podocytes were digested with 0.1% collagenase IV solution. The digestion steps were terminated by addition of complete medium. The digested tissues were percolated through a 100-µm filter, followed by centrifugation at 1,000 rpm for 5 minutes. Cells were collected and cultured using the corresponding medium. The culture medium for glomerular endothelial cells was DF12 containing 20% fetal bovine serum (FBS), penicillin, streptomycin, and vascular endothelial growth factor (VEGF). Mesangial cells were cultured in R1640 medium supplemented with 15% FBS, penicillin, streptomycin, and ITS (100×). Podocytes were cultured in KI medium and 3T3 medium at a ratio of 1:1. The KI medium contained DF12, HEPES (10 mM), penicillin, streptomycin, and ITS (100×). The 3T3 medium contained DMEM, 20% FBS, HEPES (10 mM), penicillin, streptomycin, glutamine (100×), and sodium pyruvate (1 mM).
Immunofluorescence assay
Treated cells were washed with phosphate buffered saline (PBS) solution, fixed with 4% paraformaldehyde, and incubated with the following primary antibodies: rabbit anti-CD34, anti-desmin, anti-nephrin, and anti-WT-1. Cells were then incubation with the appropriate secondary antibody conjugated to DAPI (a blue nuclear marker). Negative control staining was performed by omitting the primary antibody. The stained cells were observed using a fluorescence microscope.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells using TRIzol reagent (Thermo Fisher, Massachusetts, USA) according to the manufacturer’s instructions. Transcription of cDNA from RNA using a SuperScript III kit (Takara, Tokyo, Japan). The relative expression of the detected proteins was detected using SYBR green quantitative PCR kit (Takara, Tokyo, Japan). Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was used as the negative control. qRT-PCR was performed using the ABI Prism 7500 Fast Sequence Detection System (Applied Biosystems). The 2-ΔΔCt method was used to calculate and quantify the relative expression levels. The primer sequences used are shown in Table 1.
Table 1
| Primer ID | Sequences | Length of the primer (bp) | Length of the product (bp) | Annealing temperature (℃) |
|---|---|---|---|---|
| CXCR4 F | GGGGTCATCAAGCAAGG | 17 | 90 | 56.7 |
| CXCR4 R | CAGGCAACAGTGGAAGAAGG | 20 | ||
| GAPDH F | TCAACGGCACAGTCAAGG | 18 | 357 | 57.8 |
| GAPDH R | TGAGCCCTTCCACGATG | 17 |
CXCR4, C-X-C chemokine receptor type 4; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; F, forward; R, reverse.
Transfection
The lentivirus containing the shRNA against CXCR4 (sh-CXCR4) and the negative (blank) control (shNC) was purchased from Xiamen Lifeint Co., Ltd. The lentivirus was transfected into the cells with Lipofectamine 3000 to establish the CXCR4 knockdown cell lines.
Western blot analysis
Cells were lysed with RIPA lysis buffer containing protease and phosphatase inhibitors, and then centrifuged at 14,000 rpm for 20 minutes at 4 ℃. The total concentration of proteins was detected using the BCA assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Approximately 80 µg of protein was isolated from each sample by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Massachusetts, USA). The samples were incubated with 5% skim milk for 90 minutes to remove the non-specific binding proteins. Membranes were then incubated with primary rabbit antibodies against CXCR4, collagen IV, MMP-9, PI3K, Rac1, or VCAM-1 (1:1,000, Abcam, Massachusetts, USA) at 4 ℃ overnight. Subsequently washed with 0.1% Tris buffered saline Tween (TBST), the cell membranes were incubated with secondary antibodies and the immuno-reactive bands were observed by chemiluminescence using an ECL kit (Beyotime, Shanghai, China). The specific bands were analyzed using ImageJ software.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 software. All data are presented as mean ± standard deviation. Student’s t-tests were used for inter-group comparisons. For comparisons of 3 or more groups, one-way analysis of variance (ANOVA) was conducted, followed by the Bonferroni post-hoc test. A P value <0.05 was considered statistically significant.
Results
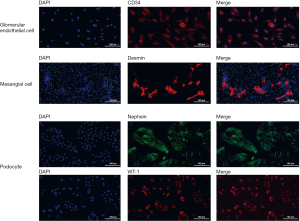
The identification of primary glomerular endothelial cells, mesangial cells, and podocytes
Primary glomerular endothelial cells, mesangial cells, and podocytes were isolated and the purity was confirmed by immunofluorescence staining for CD34, desmin, and nephrin and WT-1, respectively (Figure 1). CD34 was expressed on almost all the glomerular endothelial cells, and nephrin and WT-1 were expressed on almost all the podocytes, indicating a relatively high purity for isolated primary glomerular endothelial cells and podocytes. In contrast, desmin was only expressed on a portion of the isolated mesangial cells, indicating relatively low purity for the isolated mesangial cells.
Suppressing the activity of CXCR4 downregulated the expression of renal fibrosis-related genes in primary glomerular cells
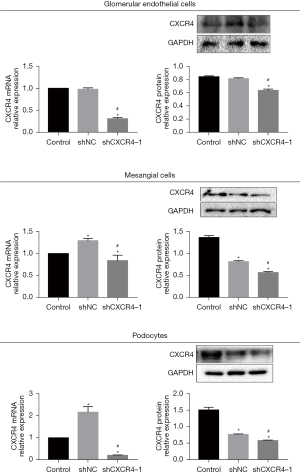
To confirm the transfection efficacy, qRT-PCR and Western blot assays were performed to assess the expression of CXCR4. As shown in Figure 2, compared to control cells, glomerular endothelial cells (HRGECs), mesangial cells (HRMCs), and podocytes (HRPs) transfected with the sh-CXCR4 showed significantly suppressed expression of CXCR4, indicating a successful knockdown of CXCR4 in all three cell lines by sh-CXCR4 (P<0.05).
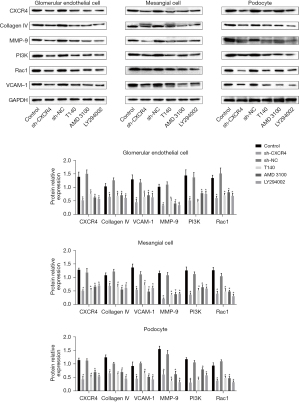
As shown in Figure 3, Western blot analyses demonstrated that the expression of CXCR4, collagen IV, MMP-9, PI3K, Rac1, and VCAM-1 were dramatically downregulated in the cell lines transfected with sh-CXCR4. Furthermore, cell lines treated with T140, AMD3100, or LY294002 also showed markedly reduced expression of CXCR4, collagen IV, MMP-9, PI3K, Rac1, and VCAM-1 compared to untreated cells. No significant differences were observed in the expression levels of these proteins between control cells and cells transfected with shNC (P<0.05).
Discussion
Tissue fibrosis is the process of repair following tissue injury, and if uncontrolled, this can contribute to sustained fibrosis which is characterized by destruction of the tissue structure and dysfunction of the organ (16). Renal fibrosis is the common denouement of various chronic renal diseases, which is mainly caused by the imbalance between the synthesis and the degradation of the extracellular matrix. Renal fibrosis is characterized by abnormal accumulation of extracellular matrix, progressive changes in the structure of the kidney, and sustained dysfunction of the kidneys. To date, there are no effective radical therapies for the treatment of clinical renal fibrosis, with current therapies concentrating on delaying the progression of the disease. Therefore, understanding the molecular mechanisms underlying renal interstitial fibrosis is crucial for the development of potential targeted therapies to reverse renal interstitial fibrosis.
CXCR4 has been shown to play an important role in the pathological process of autoimmune diseases, including the fibrosis of the lungs and liver (17). Under the normal healthy state, CXCR4 is expressed at relatively low levels in renal tissues. However, under the pathological state, such as IgA nephropathy and focal stage glomerulosclerosis, the expression of CXCR4 is significantly elevated (18). In addition, CXCR4 has been shown to promote the chronic progression of renal fibrosis (14,19). In the present study, the expression levels of fibrosis-related genes (including collagen IV, MMP-9, and VCAM-1), CXCR4, and the downstream regulatory elements (including PI3K and Rac1) were evaluated in the sh-CXCR4 transfected isolated primary glomerular endothelial cells, mesangial cells, and podocytes to verify the function of CXCR4 in the development and progression of renal fibrosis at a cellular level.
Collagen IV is the main component of glomerular basement membrane and constitutes the classical network structure in the kidney, together with laminin, proteoglycan, and paraplegic protein (20). Tissue repair and chronic inflammation can induce excessive accumulation of extracellular matrix components, such as collagen, which in turn, can be degraded by various MMPs. MMP9 is a classic MMP involved in the development and progression of fibrosis. Although the progression of fibrosis is accompanied by upregulation of MMP9, both promoting and inhibitory effects have been reported for MMP9 in the regulation of fibrosis (21,22). VCAM-1 is involved in mediating the adhesion and signal transduction of leukocytes and is highly expressed in the kidneys. VCAM-1 has also been shown to play an important role in the development of renal inflammation (23). In the present study, suppressing the activity of CXCR4 in the isolated primary glomerular endothelial cells, mesangial cells, and podocytes by incubating with CXCR4 inhibitors (T140 and AMD3100) significantly suppressed the expression of collagen IV, MMP-9, and VCAM-1. Similar results were observed when PI3K was inactivated by incubating cells with LY294002. A recent report has emphasized the positive correlation between CXCR4 and the inflammatory reaction in the kidneys, and indicated that CXCR4 inhibitors can ameliorate the symptoms of renal fibrosis by inhibiting renal inflammation through suppressing the recruitment of immune cells (24). In addition, it has also been reported that under the inflammatory state, CXCR4 located on macrophages promoted the progression of renal fibrosis via various pathways. The recruitment of macrophages could be significantly suppressed by knocking down the expression of CXCR4 on the macrophages, resulting in amelioration of renal fibrosis (25-27). In contrast to the glomerulus in the kidney, the isolated primary glomerular cells used in the present study were not influenced by other immune cells and inflammatory factors, and this is the main difference between the present study and previous reports. This investigation demonstrated that renal fibrosis-related genes could be regulated by CXCR4 and the downstream signaling pathways in the glomerulus, even in the absence of inflammation.
PI3K can be activated by the Gβγ subunit bound by CXCR4, which further activates Rac2 to form the Rac/Cdc42 complex, Finally, the chemotaxis reaction will be triggered (28). It has also been reported that P-Rex1 can be activated by the Gβγ subunit, which activates Rac1 to bind GTP independently of PI3K (29). The present study showed that the expression of PI3K and Rac1 was significantly suppressed in the primary glomerular endothelial cells, mesangial cells, and podocytes by knocking down or inactivating CXCR4, and this was similar to the effects observed with the PI3K inhibitor. We speculate that in the glomerulus, PI3K/Rac is the downstream signaling pathway of CXCR4, which regulates the expression of fibrosis-related genes.
The highest expression of CXCR4 is normally observed in the bone marrow lymphatics and the circulatory system. It is also highly expressed in the kidneys, the gastrointestinal tract, and the lungs (30). T140, an inhibitor of CXCR4, has been shown to specifically inhibit CXCR4 (31), and this may represent a promising targeted agent against CXCR4. The results of this investigation suggested that synergistic effects may be observed when combining inflammation inhibitory therapy with a CXCR4 inhibitor, and this may be a novel potential therapeutic regimen for the treatment of renal fibrosis.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81660124).
Footnote
Reporting Checklist: The authors have completed the MDAR and ARRIVE reporting checklists. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-157/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-157/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-157/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 2020052701) granted by ethics committee of Jiangxi Provincial Children’s Hospital, in compliance with Jiangxi Provincial Children’s Hospital institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- LeBleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 2013;19:1047-53. [Crossref] [PubMed]
- Campanholle G, Ligresti G, Gharib SA, et al. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 2013;304:C591-603. [Crossref] [PubMed]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011;7:684-96. [Crossref] [PubMed]
- Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol 2008;48:171-97. [Crossref] [PubMed]
- Zimmermann HW, Sterzer V, Sahin H. CCR1 and CCR2 antagonists. Curr Top Med Chem 2014;14:1539-52. [Crossref] [PubMed]
- Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest 2001;108:547-56. [Crossref] [PubMed]
- Tokuda A, Itakura M, Onai N, et al. Pivotal role of CCR1-positive leukocytes in bleomycin-induced lung fibrosis in mice. J Immunol 2000;164:2745-51. [Crossref] [PubMed]
- Ma B, Zhu Z, Homer RJ, et al. The C10/CCL6 chemokine and CCR1 play critical roles in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 2004;172:1872-81. [Crossref] [PubMed]
- Zhu Z, Ma B, Zheng T, et al. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 2002;168:2953-62. [Crossref] [PubMed]
- Chen FH, Fu SY, Yang YC, et al. Combination of vessel-targeting agents and fractionated radiation therapy: the role of the SDF-1/CXCR4 pathway. Int J Radiat Oncol Biol Phys 2013;86:777-84. [Crossref] [PubMed]
- Takabatake Y, Sugiyama T, Kohara H, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol 2009;20:1714-23. [Crossref] [PubMed]
- Ye Z, Xia Y, Zhou X, et al. CXCR4 inhibition attenuates calcium oxalate crystal deposition-induced renal fibrosis. Int Immunopharmacol 2022;107:108677. [Crossref] [PubMed]
- Ge G, Zhang H, Li R, et al. The Function of SDF-1-CXCR4 Axis in SP Cells-Mediated Protective Role for Renal Ischemia/Reperfusion Injury by SHH/GLI1-ABCG2 Pathway. Shock 2017;47:251-9. [Crossref] [PubMed]
- Yuan A, Lee Y, Choi U, et al. Chemokine receptor Cxcr4 contributes to kidney fibrosis via multiple effectors. Am J Physiol Renal Physiol 2015;308:F459-72. [Crossref] [PubMed]
- Zou XF, Gu JH, Cui ZL, et al. CXC Chemokine Receptor Type 4 Antagonism Ameliorated Allograft Fibrosis in Rat Kidney Transplant Model. Exp Clin Transplant 2017;15:448-52. [PubMed]
- Kuma A, Tamura M, Otsuji Y. Mechanism of and therapy for kidney fibrosis. J UOEH 2016;38:25-34. [Crossref] [PubMed]
- Chong BF, Mohan C. Targeting the CXCR4/CXCL12 axis in systemic lupus erythematosus. Expert Opin Ther Targets 2009;13:1147-53. [Crossref] [PubMed]
- Lotan D, Sheinberg N, Kopolovic J, et al. Expression of SDF-1/CXCR4 in injured human kidneys. Pediatr Nephrol 2008;23:71-7. [Crossref] [PubMed]
- Wu CH, Song JS, Chang KH, et al. Stem cell mobilizers targeting chemokine receptor CXCR4: renoprotective application in acute kidney injury. J Med Chem 2015;58:2315-25. [Crossref] [PubMed]
- Rubel D, Stock J, Ciner A, et al. Antifibrotic, nephroprotective effects of paricalcitol versus calcitriol on top of ACE-inhibitor therapy in the COL4A3 knockout mouse model for progressive renal fibrosis. Nephrol Dial Transplant 2014;29:1012-9. [Crossref] [PubMed]
- Prakobwong S, Yongvanit P, Hiraku Y, et al. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer 2010;127:2576-87. [Crossref] [PubMed]
- Wang Q, Diao X, Sun J, et al. Regulation of VEGF, MMP-9 and metastasis by CXCR4 in a prostate cancer cell line. Cell Biol Int 2011;35:897-904. [Crossref] [PubMed]
- Domanski L, Kłoda K, Pawlik A, et al. Correlation between ICAM1 and VCAM1 gene polymorphisms and histopathological changes in kidney allograft biopsies. Arch Med Sci 2013;9:276-82. [Crossref] [PubMed]
- Wan X, Xia W, Gendoo Y, et al. Upregulation of stromal cell-derived factor 1 (SDF-1) is associated with macrophage infiltration in renal ischemia-reperfusion injury. PLoS One 2014;9:e114564. [Crossref] [PubMed]
- Nishida M, Fujinaka H, Matsusaka T, et al. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest 2002;110:1859-68. [Crossref] [PubMed]
- Ikezumi Y, Suzuki T, Karasawa T, et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology 2011;58:198-210. [Crossref] [PubMed]
- Toki D, Zhang W, Hor KL, et al. The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant 2014;14:2126-36. [Crossref] [PubMed]
- Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 2010;16:2927-31. [Crossref] [PubMed]
- Lopez-Haber C, Barrio-Real L, Casado-Medrano V, et al. Heregulin/ErbB3 Signaling Enhances CXCR4-Driven Rac1 Activation and Breast Cancer Cell Motility via Hypoxia-Inducible Factor 1α. Mol Cell Biol 2016;36:2011-26. [Crossref] [PubMed]
- Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [Crossref] [PubMed]
- Gravel S, Malouf C, Boulais PE, et al. The peptidomimetic CXCR4 antagonist TC14012 recruits beta-arrestin to CXCR7: roles of receptor domains. J Biol Chem 2010;285:37939-43. [Crossref] [PubMed]


