
A systematic review and meta-analysis on the efficacy and safety of dexmedetomidine combined with sevoflurane anesthesia on emergence agitation in children
Introduction
Emergence agitation usually occurs acutely during anesthesia recovery, and it appears about 15 minutes after extubation in most cases (1). At present, an agitation score is used to grade emergence agitation. Severe patients will have uncontrollable and unstoppable crying, severe agitation and disorientation, and require drug intervention, which is detrimental to the postoperative recovery of pediatric patients and may lead to other complications. Sevoflurane is the latest high-efficiency inhalation anesthetic introduced in clinical practice. It is a colorless, transparent, fragrant, nonirritating volatile liquid with the advantages of a small blood/gas distribution coefficient, a fast drug effect, a short recovery time, and low liver toxicity (2). Therefore, it is widely used in the induction and maintenance of pediatric anesthesia. However, the incidence of emergence agitation after sevoflurane anesthesia is high at 10–80% (3-5).
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist that can activate presynaptic α2 receptors, thereby inhibiting the release of norepinephrine, terminating pain signal transmission, and preventing agitation-induced pain stimulation. It can combine with α2 receptor in spinal cord to exert analgesic and sedative effects. In addition, it can also inhibit the activity of sympathetic nerve, thus lowering blood pressure and heart rate, maintaining the stability of hemodynamics during anesthesia, and having the functions of sedation, analgesia, anti-anxiety and inhibition of sympathetic nerve excitement (6-8). The terminal half-life of the drug in pediatric patients is about 120 minutes, the inhibitory effect on the respiratory system is low, and increasing the dose will not enhance the respiratory depression effect. In addition, there is very little drug dependence, no rapid drug resistance, and good clinical safety. The blood pressure, heart rate, and blood oxygen saturation of children remain stable (9). Most importantly, dexmedetomidine can effectively target various anesthesia-related factors at the time of emergence and can significantly reduce the occurrence of emergence agitation after surgery.
Emergence agitation after sevoflurane anesthesia shows a higher incidence in children than in adults. Articles have shown that sevoflurane can increase the incidence of emergence agitation in children when compared with propofol (10). In addition, meta-analysis showed that the incidence of restlessness in the wake-up period of sevoflurane anesthesia was lower than that of halothane (11). To date, the etiology of emergence agitation is still unclear. Risk factors may include pain, age, quick awakening, preoperative anxiety, type of surgery, personality, and inhaled anesthetics. The use of propofol, α2 receptor agonists, midazolam, and opioids can reduce the incidence of emergence agitation. However, the research conclusion about whether dexmedetomidine can reduce the incidence of restlessness in children during the recovery period of sevoflurane anesthesia is not uniform. The aim of this meta-analysis was to comprehensively and quantitatively analyze the effect of dexmedetomidine on emergence agitation after sevoflurane anesthesia in children through a comprehensive search of relevant studies. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-172/rc).
Methods
Article retrieval
Articles were retrieved from PubMed, Embase, MEDLINE, Science Direct, The Cochrane Library, the Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, the Chinese Science and Technology Periodical Database, and the Chinese BioMedical Literature Database (CBM). Relevant articles on randomized controlled trials (RCTs) of dexmedetomidine in preventing emergence agitation after sevoflurane anesthesia in children were retrieved from the establishment of the database to October 15, 2021. Professional journals were also manually searched to avoid omissions. If the relevant data in the included articles could not be obtained from the text, the corresponding author was contacted. The search strategy was as follows. The English search keywords included “dexmedetomidine”, “children”, “sevoflurane”, and “emergence agitation”. The Chinese search keywords included “dexmedetomidine”, “children”, “sevoflurane”, and “emergence agitation”. Multiple retrievals were carried out using search engines, and the keywords were freely combined to obtain all relevant articles. The quality of the included articles was assessed using RevMan 5.3 software provided by the Cochrane Collaboration (London, UK).
Inclusion and exclusion criteria of articles
The inclusion criteria were as follows: (I) the study was an RCT of dexmedetomidine used to prevent emergence agitation after sevoflurane anesthesia in children; (II) the subjects of the study were children (under 18 years old) who underwent general anesthesia during the elective period; (III) the experimental group was given intranasal dexmedetomidine, the control group was intranasally administered with a placebo, and sevoflurane was used to induce or maintain anesthesia in all children during the operation; and (IV) the outcome indicators included incidence of emergence agitation, time of awakening, duration of postanesthesia care unit (PACU) stay, incidence of analgesic rescue, and incidence of nausea and vomiting.
The exclusion criteria were as follows: (I) non-RCTs such as reviews, letters, comments, and conference abstracts; (II) children with central nervous system disorders, congenital diseases, or liver and kidney dysfunction; (III) articles with samples older than 18 years; and (IV) repeatedly published articles with incomplete original data.
Outcome indicators
The outcome indicators included incidence of emergence agitation, time of awakening, duration of PACU stay, incidence of analgesic rescue, and incidence of nausea and vomiting.
Data extraction
Two professionals used Microsoft Excel (Microsoft, Redmond, WA, USA) to independently screen the articles, extract data according to the inclusion and exclusion criteria, and cross-check the final results. Any disagreements were solved through discussion. The extracted data included (I) the basic information of the included articles (title, first author, publication time, country, publication journal, and literature source); (II) the basic characteristics of the research subjects (gender ratio, age, and sample size in the experimental group and the control group); and (III) outcome indicators (incidence of emergence agitation, time of awakening, PACU stay time, incidence of analgesic rescue, and incidence of nausea and vomiting).
Risk-of-bias assessment
The articles included were independently evaluated and cross-checked by 2 professionals in strict accordance with the 5 evaluation criteria for RCTs in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.0.1). Any disagreements were solved through discussion. The evaluation criteria were as follows: (I) whether the random sequence generation method was correct; (II) whether the allocation concealment was strictly implemented; (III) whether the researchers applied a blinding method; (IV) whether there was dropout or loss to follow-up, and whether the outcome data were complete; and (V) whether the number of patients in each group and their age were comparable, whether there was selection bias, and whether there was a chance effect and its magnitude. Each article was assessed as “Low Risk”, “High Risk”, and “Unclear Risk” according to the specific circumstances of the included studies.
Statistical methods
The risk-of-bias assessment of the included articles was conducted using the risk-of-bias assessment chart in Rev Man 5.3. Measurement data were expressed as mean difference (MD), and enumeration data were expressed as odds ratio (OR). Each effect was expressed using a 95% CI. Heterogeneity among the articles was assessed using the chi-square (Chi2) test and the I2 test. When there is no heterogeneity (P≥0.1, I2≤50%), the fixed effect model is adopted. When there is heterogeneity (P<0.1, I2>50%), the random effect model is adopted. An inverted funnel was used to test for publication bias. When the heterogeneity comes from low-quality research, the sensitivity analysis of meta-analysis results will be carried out. P<0.05 indicates that there is a statistical difference.
Sensitivity analysis
The sensitivity analysis of efficacy indicators was performed by changing the effect model [random-effects model (REM) or fixed-effects model (FEM)] to assess the reliability of the conclusions obtained.
Results
Retrieval results and basic information of the included articles
A total of 226 articles were obtained by searching the databases. Duplicate publications (n=26) and unqualified articles (n=49) were excluded, and a further 24 articles were excluded due to other reasons. The remaining 127 articles were selected for the meta-analysis. After abstracts and titles were read, 37 articles were removed, and 90 articles were left. A further 58 research reports and reviews were then excluded, leaving 32 articles. After the full texts were read, 8 articles with incorrect research types were excluded, and 4 articles were excluded because the diagnostic data of the study could not be extracted. A further 4 articles were excluded that did not mention children who underwent elective surgery. A total of 16 articles were finally included in the meta-analysis. Figure 1 is a flow chart of the literature retrieval process.
The quality evaluation results showed that all 7 literatures were of high quality, and all 9 literatures were of medium quality. In the 16 articles that met the inclusion criteria, there were 1,585 patients in total, including 799 in the experimental group and 786 in the control group. Fifteen articles described the incidence of emergence agitation, 3 described the time of awakening, 6 described the duration of PACU stay, 3 described the incidence of analgesic rescue, and 8 described the incidence of nausea and vomiting. Table 1 lists the basic characteristics of the included articles.
Table 1
| First author | Year of publication | Number of cases | Intervention | Outcome indicators | |||
|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | ||||
| Abdelaziz (12) | 2016 | 33 | 32 | 1 μg/kg dexmedetomidine | 1 mL of normal saline | Incidence of emergence agitation, time of awakening, incidence of analgesic rescue, duration of PACU stay, and incidence of nausea and vomiting | |
| Cai (13) | 2021 | 46 | 37 | 2 μg/kg dexmedetomidine | 1 μg/kg midazolam | Incidence of emergence agitation and vomiting | |
| Chen (14) | 2013 | 27 | 24 | 1 μg/kg dexmedetomidine | 50 mL of normal saline | Incidence of emergence agitation, and incidence of nausea and vomiting | |
| El-Hamid (15) | 2017 | 43 | 43 | 1 μg/kg intranasal dexmedetomidine | 0.9% intranasal saline | Incidence of emergence agitation and duration of PACU stay | |
| Guler (16) | 2005 | 30 | 30 | 0.5 μg/kg dexmedetomidine | Placebo | Incidence of emergence agitation, and incidence of nausea and vomiting | |
| Isik (17) | 2006 | 21 | 21 | 1 μg/kg dexmedetomidine | 10 mL of normal saline | Incidence of emergence agitation, time of awakening, and incidence of nausea and vomiting | |
| Kim (18) | 2014 | 20 | 20 | 1 μg/kg dexmedetomidine | Isometric saline | Incidence of emergence agitation | |
| Lili (19) | 2012 | 30 | 30 | 0.5 μg/kg dexmedetomidine | 10 mL of normal saline | Incidence of emergence agitation | |
| Lin-A (20) | 2016 | 30 | 30 | 1 μg/kg dexmedetomidine | Isometric saline | Duration of PACU stay, and incidence of analgesic rescue | |
| Lin-B | 30 | 30 | 2 μg/kg dexmedetomidine | Isometric saline | |||
| Liu (21) | 2021 | 168 | 168 | 1 mL dexmedetomidine +4% sevoflurane | 2 mg/kg ketamine | Incidence of emergence agitation and vomiting | |
| Pestieau-A (22) | 2011 | 23 | 27 | 1 μg/kg dexmedetomidine | Isometric saline | Incidence of emergence agitation, and incidence of analgesic rescue | |
| Pestieau-B | 28 | 27 | 2 μg/kg dexmedetomidine | Isometric saline | |||
| Sato (23) | 2010 | 39 | 42 | 0.25 μg/kg dexmedetomidine | Isometric saline | Incidence of emergence agitation | |
| Song-A (24) | 2016 | 25 | 25 | 0.25 μg/kg dexmedetomidine | Isometric saline | Incidence of emergence agitation, duration of PACU stay, and incidence of nausea and vomiting | |
| Song-B | 25 | 25 | 0.5 μg/kg dexmedetomidine | Isometric saline | |||
| Song-C | 28 | 25 | 1 μg/kg dexmedetomidine | Isometric saline | |||
| Sun-A (25) | 2017 | 23 | 24 | 0.25 μg/kg dexmedetomidine | Isometric saline | Incidence of emergence agitation, time of awakening, and duration of PACU stay | |
| Sun-B | 25 | 24 | 0.5 μg/kg dexmedetomidine | Isometric saline | |||
| Sun-C | 25 | 24 | 1 μg/kg dexmedetomidine | Isometric saline | |||
| Yao-A (26) | 2015 | 30 | 29 | 1 μg/kg dexmedetomidine | Isometric saline | Duration of PACU stay | |
| Yao-B | 30 | 29 | 2 μg/kg dexmedetomidine | Isometric saline | |||
| Zhang (27) | 2022 | 20 | 20 | 2 μg/kg dexmedetomidine | Isometric saline | Incidence of emergence agitation and vomiting | |
Note: PACU, postanesthesia care unit; A, B, C in the table represent different groups in the same article.
Assessment results on risk of bias in the included articles
Figures 2,3 show the risk of bias assessment chart and the summary chart drawn by RevMan 5.3 software. Of the 16 RCTs, 14 described the generation of random sequences in detail, 8 described allocation concealment in detail, no patient blinding was described due to different surgical methods, 8 articles used operator blinding, and all 16 articles had complete outcome measures. Excepting patient blinding, all other risks of bias were low.

Meta-analysis results of the incidence of emergence agitation
A total of 16 articles (12-27) analyzed the incidence of emergence agitation in children anesthetized with dexmedetomidine combined with sevoflurane. Subgroup analyses were performed according to different doses of dexmedetomidine. Three articles used 0.25 µg/kg dexmedetomidine, with 90 cases in the experimental group and 91 cases in the control group. A FEM was used to analyze this subgroup (Figure 4), and the heterogeneity test showed no heterogeneity among the 3 articles [Chi2=0.12, degree of freedom (df) =2, I2=0%, P=0.94]. The incidence of postoperative emergence agitation in the 0.25 µg/kg dexmedetomidine group was significantly lower than that in the control group, and the difference was statistically significant (OR =0.44, 95% CI: 0.21, 0.93, P=0.03).

Five articles used 0.5 µg/kg dexmedetomidine, with 133 cases in the experimental group and 129 cases in the control group. A FEM was used to analyze this subgroup (Figure 5), and the heterogeneity test showed no heterogeneity among the articles (Chi2 =2.96, df =4, I2=0%, P=0.56). The incidence of postoperative emergence agitation in the 0.5 µg/kg dexmedetomidine group was significantly lower than that in the control group, and the difference was statistically significant (OR =0.22, 95% CI: 0.13, 0.40, P<0.00001).
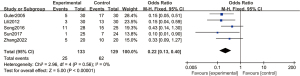
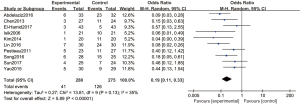
Ten articles used 1 µg/kg dexmedetomidine, with 280 cases in the experimental group and 275 cases in the control group. A FEM was used to analyze this subgroup (Figure 6), and the heterogeneity test showed no heterogeneity among the articles (Chi2 =13.81, df =9, I2=35%, P=0.13). The incidence of postoperative emergence agitation in the 1 µg/kg dexmedetomidine group was significantly lower than that in the control group, and the difference was statistically significant (OR =0.19, 95% CI: 0.12, 0.28, P<0.00001).
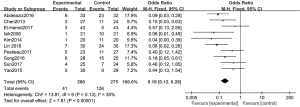
Four articles used 2 µg/kg dexmedetomidine, with 134 cases in the experimental group and 123 cases in the control group. A REM was used to analyze this subgroup (Figure 7), and there was significant heterogeneity among the articles (Chi2=18.68, df =3, I2=84%, P=0.0003). There was no statistically significant difference in the incidence of emergence agitation between the 2 µg/kg dexmedetomidine group and the control group (OR =0.29, 95% CI: 0.06, 1.35, P=0.12).
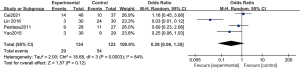
Figure 8 is a funnel chart of the incidence of agitation of dexmedetomidine at 1 µg/kg. The circles representing the included articles were concentrated near the midline and were basically symmetrical. This suggested that there was no publication bias in the results of this meta-analysis.
Meta-analysis of time of awakening
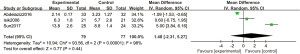
A total of 3 articles analyzed the recovery time of postoperative children. There were 79 cases in the experimental group and 77 cases in the control group. A REM was used to analyze time of awakening (Figure 9), and the heterogeneity test showed heterogeneity among the articles (Chi2=93.56, df =2, I2=98%, P<0.00001). There was no significant difference in recovery time between the experimental group and the control group (MD =1.48, 95% CI: −2.31, 5.27, Z =0.77, P=0.44). Figure 10 is a funnel plot of time of awakening. The circles representing the included articles were concentrated near the midline and were basically symmetrical. This suggested that there was no publication bias in the results of this meta-analysis.
Meta-analysis results of duration of PACU stay
A total of 6 articles analyzed duration of PACU stay. Subgroup analyses were performed according to different doses of dexmedetomidine. Six articles used 1 µg/kg dexmedetomidine, with 169 cases in the experimental group and 167 cases in the control group. A FEM was used to analyze duration of PACU stay using 1 µg/kg dexmedetomidine (Figure 11), and the heterogeneity test showed no heterogeneity among the articles (Chi2=5.31, df =5, I2=6%, P=0.38). The duration of PACU stay in the experimental group was significantly longer than that in the control group, and the difference was statistically significant (MD =1.09, 95% CI: 0.26, 1.92, Z =2.58, P=0.010).
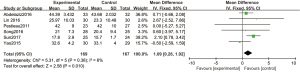
Three articles used 2 µg/kg dexmedetomidine, with 88 cases in the experimental group and 86 cases in the control group. A REM was used to analyze duration of PACU stay using 2 µg/kg dexmedetomidine (Figure 12), and the heterogeneity test showed heterogeneity among the articles (Chi2=60.80, df =2, I2=97%, P<0.00001). The duration of PACU stay in the experimental group was not significantly different to that in the control group (MD =9.99, 95% CI: −4.31, 24.30, Z=1.37, P=0.17).

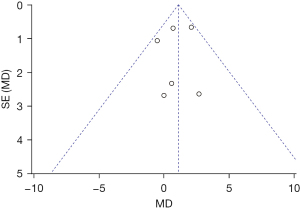
Figure 13 is a funnel plot of the duration of PACU stay in the 1 µg/kg dexmedetomidine group. The circles representing the included articles were concentrated near the midline and were basically symmetrical. This suggested that there was no publication bias in the results of this meta-analysis.
Results of a meta-analysis of the incidence of analgesic rescue
A total of 3 articles analyzed the incidence of analgesic rescue. There were 86 cases in the experimental group and 89 cases in the control group. A FEM was used to analyze the incidence of analgesic rescue (Figure 14), and the heterogeneity test showed no heterogeneity among the articles (Chi2=1.04, df =2, I2=0%, P=0.60). The incidence of analgesic rescue in the experimental group was significantly lower than that in the control group, and the difference was statistically significant (OR =0.29, 95% CI: 0.13, 0.63, Z=3.13, P=0.002).

Figure 15 is a funnel plot of the incidence of analgesic rescue. The circles representing the included articles were concentrated near the midline and were basically symmetrical. This suggested that there was no publication bias in the results of this meta-analysis.
Meta-analysis results on incidence of postoperative nausea and vomiting
A total of 8 articles analyzed the incidence of postoperative nausea and vomiting. There were 370 cases in the experimental group and 360 cases in the control group. A FEM was used to analyze the incidence of postoperative nausea and vomiting (Figure 16), and the heterogeneity test showed no heterogeneity among the articles (Chi2=2.38, df =7, I2=0%, P=0.94). The incidence of postoperative nausea and vomiting in the experimental group was significantly lower than that in the control group, and the difference was statistically significant (OR =0.33, 95% CI: 0.20, 0.55, Z=4.29, P<0.0001).
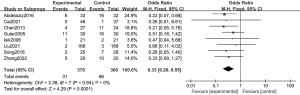
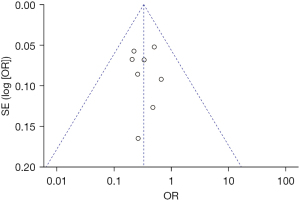
Figure 17 is a funnel plot of the incidence of postoperative nausea and vomiting. The circles representing the included articles were concentrated near the midline and were basically symmetrical. This suggested that there was no publication bias in the results of this meta-analysis.
Sensitivity analysis
A sensitivity analysis was performed by changing the analytical model (FEM to REM). Figure 18 is a forest plot of the incidence of emergence agitation with 1 µg/kg dexmedetomidine. The results were OR =0.19, 95% CI: 0.11, 0.33, Z=5.89, and P<0.00001. This suggested that the conclusions obtained by applying different analysis models were consistent, and the results had good stability.
Discussion
Emergence agitation is a common postoperative complication that predominantly affects school-aged children aged 3–5 (28). Epidemiological data show that the incidence of emergence agitation in children is about 12%, while the incidence in adults is about 5% (29). Typically occurring within the first 15 minutes of awakening after anesthesia, emergence agitation involves a mental state in which consciousness and behavior are separated. The main clinical manifestations in children are crying, restlessness, moaning, wiggling, inability to be comforted, incoherent speech, disorientation, and inability to recognize people or objects (30,31). The occurrence of postoperative restlessness is related to many factors, such as personality, pain, age, quick awakening, preoperative anxiety, and surgical type (32). Rapid awakening is also one of the risk factors affecting restlessness during awakening. When the concentration of inhaled narcotic drugs drops rapidly in a short period of time, if the timing of extubation is not appropriate, the child’s awareness and feeling have been restored, but his consciousness has not yet recovered, and he is in a state of high sensitivity to external stimuli, which will lead to restlessness during awakening (33). The clinical harm of emergence agitation can be severe, and it has a huge impact on the patients themselves, as some children require treatment after surgery. Some children are prone to violence, leading to increased blood pressure and heart rate, which is detrimental to hemodynamic stability and may lead to cardiovascular diseases (34). In addition, involuntary movements of the child may cause the surgical incision to rupture and bleed, leading to surgical site infection and nonhealing. The occurrence of emergence agitation also increases the risk of self-injury in children, prolongs the hospital stay, and increases additional medical expenses. Therefore, it is necessary to choose appropriate and targeted drugs to effectively prevent the occurrence of postoperative emergence agitation according to the individual condition of the patient.
Sevoflurane is widely used in pediatric anesthesia due to its low blood gas partition coefficient, rapid induction of anesthesia, and quick recovery after surgery. However, the incidence of emergence agitation after sevoflurane anesthesia is high. Cohen et al. (35) reported that children’s self-control ability was low. When two groups of children inhaled propofol and sevoflurane respectively, the extubation time and recovery were similar, but the incidence of sevoflurane awakening agitation period was significantly higher than that of propofol. When the cerebral cortex is still in a state of inhibition but the subcortical center has been liberated, a state of separation occurs, which makes the child more sensitive to the surrounding environment. When this state is coupled with pain stimuli, emergence agitation may occur very easily.
Dexmedetomidine, an adrenal α2 receptor agonist, is widely used for anesthesia and sedation during surgery. In recent years, articles have found that dexmedetomidine can significantly reduce the incidence of anesthesia emergence agitation in children (36). The results of this meta-analysis showed that the incidence of sevoflurane anesthesia emergence agitation in children was significantly reduced in the dexmedetomidine experimental group. The subgroup analyses showed that 0.25, 0.5, and 1 µg/kg of dexmedetomidine reduced the incidence of emergence agitation in the experimental group compared with that in the control group (P<0.05), which was consistent with the results of Sun et al. (37).
Dexmedetomidine has sedative, analgesic, and anxiolytic effects, as well as a certain antisympathetic effect, and can significantly reduce the release of catecholamines in the perioperative period, maintain hemodynamic stability, and reduce the perioperative stress response. Cravero et al. (38) found that pain alone did not cause emergence agitation, but that poor postoperative pain control may lead to emergence agitation, so postoperative pain may be a factor in the incidence of emergence agitation. Dexmedetomidine can exert analgesic effect at spinal level, and reduce the dosage of opioid analgesics after operation, which indicates that dexmedetomidine may reduce the incidence of restlessness during awakening through analgesic effect. Our meta-analysis showed that the incidence of analgesic rescue in the dexmedetomidine experimental group was significantly lower than that in the control group (P<0.05).
Postoperative nausea and vomiting are common complications after surgical procedures. Our results showed that the incidence of nausea and vomiting in the experimental group was significantly lower than that in the control group (P<0.05), which was consistent with the findings of Xu et al. (39). This suggests that the synergistic effect of dexmedetomidine and anesthetics can significantly reduce the dosage of propofol and opioids, thereby reducing the incidence of nausea and vomiting.
The sensitivity analysis showed that the conclusions obtained by applying different analysis models in the meta-analysis were consistent, and the results had good stability. However, there were still some limitations in this meta-analysis. Each study was based on a different study protocol, and there were differences in the timing and doses of dexmedetomidine. Other potential sources of heterogeneity included differences in the age of the children, the severity of the underlying disease, and the type of surgery.
Conclusions
This meta-analysis screened relevant articles to explore the effectiveness of dexmedetomidine in preventing sevoflurane anesthesia emergence agitation in children. The results confirmed that dexmedetomidine can reduce the incidence of emergence agitation, postoperative analgesic rescue, and nausea and vomiting in children after sevoflurane anesthesia. Therefore, this meta-analysis provided scientific evidence for the clinical prevention of emergence agitation using dexmedetomidine.
However, due to the small sample size of the included studies, the test performance of this meta-analysis may be reduced. Therefore, further studies with larger sample sizes need to be conducted to confirm the results presented here, and this research should be conducted in strict compliance with the standards of RCTs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-172/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-172/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- He L, Wang X, Zheng S. Effects of dexmedetomidine on sevoflurane requirement for 50% excellent tracheal intubation in children: a randomized, double-blind comparison. Paediatr Anaesth 2014;24:987-93. [Crossref] [PubMed]
- Zhang Y, Li M, Cui E, et al. Dexmedetomidine attenuates sevoflurane induced neurocognitive impairment through α2 adrenoceptors. Mol Med Rep 2021;23:38. [PubMed]
- Zhu M, Wang H, Zhu A, et al. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PLoS One 2015;10:e0123728. [Crossref] [PubMed]
- Fang XZ, Gao J, Ge YL, et al. Network Meta-Analysis on the Efficacy of Dexmedetomidine, Midazolam, Ketamine, Propofol, and Fentanyl for the Prevention of Sevoflurane-Related Emergence Agitation in Children. Am J Ther 2016;23:e1032-42. [Crossref] [PubMed]
- Ibacache ME, Muñoz HR, Brandes V, et al. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg 2004;98:60-3. [Crossref] [PubMed]
- Tan D, Xia H, Sun S, et al. Effect of ancillary drugs on sevoflurane related emergence agitation in children undergoing ophthalmic surgery: a Bayesian network meta-analysis. BMC Anesthesiol 2019;19:138. [Crossref] [PubMed]
- Costi D, Cyna AM, Ahmed S, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 2014;CD007084. [Crossref] [PubMed]
- Sun M, Dong Y, Li M, et al. Dexmedetomidine and Clonidine Attenuate Sevoflurane-Induced Tau Phosphorylation and Cognitive Impairment in Young Mice via α-2 Adrenergic Receptor. Anesth Analg 2021;132:878-89. [Crossref] [PubMed]
- Gupta N, Rath GP, Prabhakar H, et al. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol 2013;25:271-8. [Crossref] [PubMed]
- Di M, Yang Z, Qi D, et al. Intravenous dexmedetomidine pre-medication reduces the required minimum alveolar concentration of sevoflurane for smooth tracheal extubation in anesthetized children: a randomized clinical trial. BMC Anesthesiol 2018;18:9. [Crossref] [PubMed]
- Tang W, He D, Liu Y. Effect of Dexmedetomidine in children undergoing general anaesthesia with sevoflurane: a meta-analysis and systematic review. J Int Med Res 2020;48:300060520927530. [Crossref] [PubMed]
- Abdelaziz HMM, Bakr RH, Kasem AA. Effect of intranasal dexmedetomidine or intranasal midazolam on prevention of emergence agitation in pediatric strabismus surgery: A randomized controlled study. Egypt J Anaesth 2016;32:285-91. [Crossref]
- Cai YH, Wang CY, Li Y, et al. Comparison of the Effects of Oral Midazolam and Intranasal Dexmedetomidine on Preoperative Sedation and Anesthesia Induction in Children Undergoing Surgeries. Front Pharmacol 2021;12:648699. [Crossref] [PubMed]
- Chen JY, Jia JE, Liu TJ, et al. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth 2013;60:385-92. [Crossref] [PubMed]
- El-Hamid AMA, Yassin HM. Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy and/or adenoidectomy. Saudi J Anaesth 2017;11:137-43. [Crossref] [PubMed]
- Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth 2005;15:762-6. [Crossref] [PubMed]
- Isik B, Arslan M, Tunga AD, et al. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth 2006;16:748-53. [Crossref] [PubMed]
- Kim NY, Kim SY, Yoon HJ, et al. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J 2014;55:209-15. [Crossref] [PubMed]
- Lili X, Jianjun S, Haiyan Z. The application of dexmedetomidine in children undergoing vitreoretinal surgery. J Anesth 2012;26:556-61. [Crossref] [PubMed]
- Lin Y, Chen Y, Huang J, et al. Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J Clin Anesth 2016;33:289-95. [Crossref] [PubMed]
- Liu W, Yu Q, Jiang R, et al. Comparison of Low-Dose Sevoflurane Inhalation With Intranasal Ketamine as Rescue Sedation After Intranasal Dexmedetomidine Failure in Outpatient Children Undergoing MRI: A Randomized Control Trial. J Perianesth Nurs 2021;36:492-8. [Crossref] [PubMed]
- Pestieau SR, Quezado ZM, Johnson YJ, et al. The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatr Anaesth 2011;21:1128-35. [Crossref] [PubMed]
- Sato M, Shirakami G, Tazuke-Nishimura M, et al. Effect of single-dose dexmedetomidine on emergence agitation and recovery profiles after sevoflurane anesthesia in pediatric ambulatory surgery. J Anesth 2010;24:675-82. [Crossref] [PubMed]
- Song IA, Seo KS, Oh AY, et al. Dexmedetomidine Injection during Strabismus Surgery Reduces Emergence Agitation without Increasing the Oculocardiac Reflex in Children: A Randomized Controlled Trial. PLoS One 2016;11:e0162785. [Crossref] [PubMed]
- Sun Y, Li Y, Sun Y, et al. Dexmedetomidine Effect on Emergence Agitation and Delirium in Children Undergoing Laparoscopic Hernia Repair: a Preliminary Study. J Int Med Res 2017;45:973-83. [Crossref] [PubMed]
- Yao Y, Qian B, Lin Y, et al. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr Anaesth 2015;25:492-8. [Crossref] [PubMed]
- Zhang YZ, Wei XL, Tang B, et al. The Effects of Different Doses of Alfentanil and Dexmedetomidine on Prevention of Emergence Agitation in Pediatric Tonsillectomy and Adenoidectomy Surgery. Front Pharmacol 2022;13:648802. [Crossref] [PubMed]
- Hadi SM, Saleh AJ, Tang YZ, et al. The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int J Pediatr Otorhinolaryngol 2015;79:671-6. [Crossref] [PubMed]
- Patel A, Davidson M, Tran MC, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg 2010;111:1004-10. [Crossref] [PubMed]
- Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: A comparison of dexmedetomidine and propofol. Saudi J Anaesth 2013;7:296-300. [Crossref] [PubMed]
- Xu J, Deng XM, Wei LX, et al. Effects of Two Intranasal Dexmedetomidine Doses as Premedication on Sevoflurane EC50 for Successful Laryngeal Mask Airway Placement in Children. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016;38:627-31. [PubMed]
- Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology 2008;109:225-32. [Crossref] [PubMed]
- Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 2010;104:216-23. [Crossref] [PubMed]
- Kanaya A, Kuratani N, Satoh D, et al. Lower incidence of emergence agitation in children after propofol anesthesia compared with sevoflurane: a meta-analysis of randomized controlled trials. J Anesth 2014;28:4-11. [Crossref] [PubMed]
- Cohen IT, Finkel JC, Hannallah RS, et al. Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: a comparison with propofol. Paediatr Anaesth 2003;13:63-7. [Crossref] [PubMed]
- Wei LX, Deng XM, Wang L, et al. Effect of Intravenous Dexmedetomidine Injection(1 μg/kg)on the Intubating Conditions without Muscle Relaxants in Children after Inhalation Induction with Sevoflurane. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017;39:465-70. [PubMed]
- Sun L, Guo R, Sun L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand 2014;58:642-50. [Crossref] [PubMed]
- Cravero JP, Beach M, Thyr B, et al. The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth Analg 2003;97:364-7. [Crossref] [PubMed]
- Xu C, Zhang Y, Zhang T, et al. Efficacy and Safety of Intranasal Dexmedetomidine During Recovery From Sevoflurane Anesthesia in Children: A Systematic Review and Meta-analysis. Clin Neuropharmacol 2021;44:157-68. [Crossref] [PubMed]







