
Atypical CT findings of renal neuroblastoma: a case report
Introduction
Neuroblastoma (NB) is a highly malignant tumor of the ectoderm of sympathetic nerve cells, often occurring in sympathetic nerve tissues and originating from neural crest cells. It is one of the most common malignant tumors in children, accounting for approximately 10% (1). More than 70% of NBs occur in children under 5 years old, and the incidence is higher in males. Furthermore, it can occur in any part of the sympathetic nerve plexus distribution; about 65% of NBs are located in the abdomen, of which approximately 70% are located in the adrenal gland. Most of the remaining 30% of cases originate from the sympathetic trunk and the anterior sacral area near the spine, and occasionally from the abdominal trunk or the para-aorta (2), and is less common in the kidney. Radiological examinations [e.g., ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) examinations] play a significant role in the diagnosis of renal NB, the typical CT signs of renal NB are large or huge lobulated soft tissue mass in the retroperitoneum, with blurred borders, incomplete capsule, infiltration into the surrounding area, easy to surround and bury renal blood vessels, which is prone to necrosis, hemorrhage, cystic degeneration, and calcification, and the incidence of calcification is about 70%, mostly sandy or massive calcification (3,4). Herein, we report a case of renal NB with atypical CT findings, the purpose of which is to improve the understanding of the disease and the level of imaging diagnosis for further precise clinical treatment. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-205/rc).
Case presentation
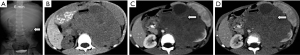
A 4-year-old male child came to our hospital for medical attention due to “abdominal distension for 1 day”. Physical examination revealed swelling on the left side of the abdomen, a locally palpable mass, and percussive pain in the left kidney area, with no other signs of discomfort. Laboratory tests and tumor markers were negative. Intravenous urography showed an enlarged left renal shadow, suggesting the high possibility of a mass (Figure 1A). Abdominal CT examination revealed a large soft tissue mass of uneven density in the left retroperitoneum, that was poorly demarcated from the left kidney and was considered to be a nephroblastoma (Figure 1B-1D).
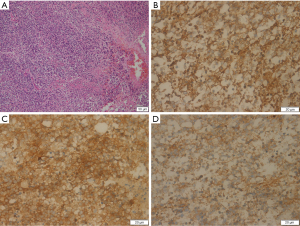
Subsequently, the patient underwent surgery to remove the tumor. During the operation, a huge tumor (about 26 cm × 20 cm × 22 cm) was observed in the upper pole of the left kidney, with local cystic degeneration. The tumor was soft, adhered to the surrounding tissues, invaded the renal capsule, and involved the renal pelvis. Intraoperatively, the tumor tissue was sent for frozen sectioning, which indicated that the tumor was malignant, so left renal radical resection was immediately performed. The left renal pedicle vessel was severed, and the left kidney and tumor tissue were removed. Postoperative immunopathology is shown in Figure 2. The neoplastic cells were round or ovoid and were fused into fragments with ill-defined boundaries and hyperchromatic nuclei. Immunohistochemical staining of the tumor cells showed positive expression of vimentin, neuron-specific enolase (NSE), KI-67, neural cell adhesion molecules (CD56), and synaptophysin (Syn), and negative expression of cytokeratin (CK), desmin, epithelial membrane antibody (EMA), WT-1, CD99, lymphocyte common antigen (LCA), and neurofilament protein (NF), etc. According to the above microscopic characteristics, a pathologic diagnosis of left renal NB (grade IV) was made, with no tumor involvement at the broken end of the ureter. In addition, further genetic examination showed that the neuron-specific enolase was increased (with a value of 33.6 ng/mL), and the N-MYC gene was amplified, but the vanilla bitter almond value was normal.
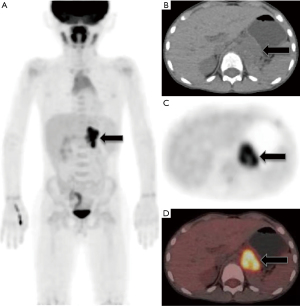
Postoperatively, the patient achieved complete remission after receiving eight courses of chemotherapy with mesna, cyclophosphamide, and irinotecan hydrochloride. Two years later, the patient consciously came to our hospital for back pain on the left side, and positron emission tomography (PET)/CT showed metastases in the left retroperitoneum (PET/CT images are shown in Figure 3), and the patient was lost to follow-up after 1 year.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Oral and written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The incidence of renal malignancy in children gradually increases with age (5), with the most common being Wilms tumor, accounting for up to 90% (6). Other primary renal tumors are relatively rare. The pathogenesis of NB originating in the kidney is still unclear, and most scholars believe that it is more likely to originate from the adrenal medulla or the sympathetic ganglion in the kidney. Renal NB usually has no clinical symptoms in the early stage; as the disease progresses, the tumor may press on the surrounding intestinal structure and induce an intestinal reaction. When the tumor invades the hilum, the patient may present painless hematuria and other non-specific manifestations. NB is a neuroendocrine tumor, which is composed of small round or oval cells of uniform size (observable under light microscopy), with large hyperchromatic nuclei. Tumor cells may be arranged in a daisy-shaped nest or rosette configuration.
The immunohistochemical results of our patient were consistent with tumor markers expressed specifically by neuroendocrine tumors, such as NSE, chromogranin A (CgA), and Syn (7). Previous studies (3,4) have suggested that typical CT signs of renal NB include large or huge lobulated soft tissue masses in the retroperitoneum, with fuzzy boundaries and an incomplete capsule, which infiltrate into the surrounding area and are easy to surround and bury renal vessels. Moreover, these masses are prone to necrosis, hemorrhage, cystic degeneration, and calcification, of which the incidence of calcification is about 2/3, and is usually sandy or massive calcification. In this case, Contrast-enhanced CT showed mild to obvious enhancement of the tumor, but no enhancement in the cystic degeneration and necrotic areas. We also found that the tumor tissue easily crossed the midline and invaded the abdominal aorta and inferior vena cava, pushing them forward. The CT findings of this patient showed no obvious calcification; therefore, the radiologist’s primary diagnosis was misdiagnosed as nephroblastoma.
NB develops rapidly and has a high degree of malignancy, which is easily transferred to the bone marrow, bone, lung, brain, and other organs in the early stage. Approximately 60% of children have metastasis at the time of diagnosis, which seriously threatens their lives. According to the characteristics of the primary tumor, lymph node involvement, invasion of adjacent structures, and the presence or absence of metastatic disease, the International Neuroblastoma Staging System (INSS) has proposed multiple treatments for NB (8). However, since renal NB is not common clinically, and clinicians are still lack research and experience, the current treatment regimen for renal NB in children generally follows that of nephroblastoma, which is the most common renal tumor in children. Surgical resection is the most effective treatment method, but in patients with late stage disease, the possibility of complete surgical resection is often reduced due to the close connection between tumor tissues and blood vessels or tumor growth around blood vessels, thus affecting the prognosis.
The role of chemotherapy in neuroendocrine tumors has been difficult to evaluate due to the rarity and biological variability of neuroendocrine tumors. Some scholars have suggested adjuvant chemotherapy for patients with locally advanced NB. The most commonly used chemotherapy drugs include cyclophosphamide, ifosfamide, cisplatin, carboplatin, etoposide, and adriamycin, etc. However, their clinical efficacy is not significant, and therefore, the survival rate of children can only be improved through early diagnosis and treatment (6,7,9,10). Our patient was at an advanced stage at the time of hospitalization; thus, despite receiving surgical resection and postoperative chemotherapy [fluorine-18 fluorodeoxyglucose (18F-FDG)], PET/CT imaging of the systemic tumor showed multiple retroperitoneal lymph node metastases 3 months after surgery, and the patient was lost to follow-up after 1 year.
Based on the above description, we analyzed the reasons why this patient was misdiagnosed as nephroblastoma before surgery: (I) nephroblastoma is the most common renal malignant tumor in children, and primary NB in the kidney is relatively rare, and thus fails to attract sufficient attention from surgeons and radiologists; (II) the CT findings of this patient were atypical, and there was no relatively specific calcification in the diagnosis of NB, and there was also less calcification in the diagnosis of nephroblastoma; and (III) NB can produce catecholamines, so most patients have significantly increased vanillylmandelic acid (VMA) in the blood and urine; however, in this case, the indicators in laboratory tests were within the normal range.
CT plays an important role in discovering renal tumors, determining the relationship between tumors and surrounding tissues, and characterizing tumors. Contrast-enhanced scanning can help determine tumor boundaries and help in qualitative diagnosis (11). According to the clinical features and CT manifestations of renal masses in children, renal NB should be differentiated from the following tumors: (I) Wilms, which is the most common renal malignancy in children. CT findings show mixed density masses from the renal parenchyma, with a few cases of hemorrhage and calcification. The calcification rate is much lower than that of NB (12,13). Also, CE-CT shows uneven enhancement of the tumor soft tissue, and “crescent” enhancement is a typical enhancement feature of Wilms. When the tumor is large, it could cross the midline and compress the surrounding great vessels, leading to its displacement under pressure, but this tumor does not wrap around the great vessels, which distinguishes it from renal NB. (II) Clear cell carcinoma of the kidney, which usually occurs in adults and may also exhibit cystic degeneration, necrosis, bleeding, or calcification. CE-CT shows significant enhancement at the renal cortical phase, while the enhancement degree of lesions at the medullary phase and delayed scan decrease rapidly, presenting a typical “fast in and fast out” performance (14,15), which differs from the progressive enhancement of renal NB. (III) Ewing’s sarcoma, which is another malignant tumor with a low incidence in the kidney, and is usually characterized by a single large, ill-defined, irregular soft-tissue mass in the kidney on CT. This mass tends to be invasive and prone to necrosis, cystic degeneration, and hemorrhage, so its density is often uneven. CE-CT shows that the enhancement degree of the tumor parenchyma is different, mainly in the form of separation, flower ring, and honeycomb, which has certain characteristics (16,17). (IV) Renal clear cell sarcoma, which has a relatively low incidence, but is more common in children. CT shows a large soft tissue mass in the kidney that is prone to hemorrhage, cystic degeneration, and necrosis. Calcification is relatively rare. CE-CT shows obvious enhancement and characteristic enhancement changes of plaques and stripes in the tumor (18). In addition, primary NB of the kidney needs to be distinguished from renal invasion by retroperitoneal tumor.
Conclusions
NB originating in the kidney is rare, and its clinical characteristics are not specific. When the CT features of renal NB are atypical, it is easily misdiagnosed as nephroblastoma. NB has a very high degree of malignancy, and even after surgical resection, the tumor is prone to relapse and distant metastasis. Therefore, understanding the characteristics of this disease is of great clinical significance for the diagnosis and early intervention in this disease. The final diagnosis of renal NB should be combined with pathology and immunohistochemistry results.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of the Peoples Republic of China, NSFC (grant number: 81571712), National Science and Technology Foundation of Zunyi City [No. HZ (2021)109], and the Zunyi Medical College Research Start Fund (No. 2018ZYFY03).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-205/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-205/coif). YT reports that the study was funded by National Science and Technology Foundation of Zunyi City [No. HZ(2021)109]. PW reports that the study was funded by Zunyi Medical College Research Start Fund (2018ZYFY03). JC reports that this study was funded by National Natural Science Foundation of the Peoples Republic of China, NSFC (grant number: 81571712). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Oral and written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lonergan GJ, Schwab CM, Suarez ES, et al. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics 2002;22:911-34. [Crossref] [PubMed]
- Rha SE, Byun JY, Jung SE, et al. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics 2003;23:29-43. [Crossref] [PubMed]
- Tang JY, Pan C, Chen J, et al. Comprehensive protocol for diagnosis and treatment of childhood neuroblastoma--results of 45 cases. Zhonghua Er Ke Za Zhi 2006;44:770-3. [PubMed]
- Jiang T, Ma L, Cheng LQ. Imaging of renal neuroblastoma: a case report. Chinese Journal of Medical Imaging. 2014;12:948-9.
- Faizan M, Sultana N, Anwar S, et al. Intrarenal neuroblastoma: a diagnostic challenge. J Coll Physicians Surg Pak 2015;25:S41-2. [PubMed]
- Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am 2015;62:225-56. [Crossref] [PubMed]
- Kushner BH, Kramer K, LaQuaglia MP, et al. Neuroblastoma in adolescents and adults: the Memorial Sloan-Kettering experience. Med Pediatr Oncol 2003;41:508-15. [Crossref] [PubMed]
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. Prog Clin Biol Res 1994;385:363-9. [PubMed]
- Yang B, Wang S, Shi PL. Application of CT in diagnosis and follow-up of neuroblastoma in children. Modern Oncology Medicine 2016;15:300-2.
- Chen LY, Wang YZ, Zou J. Role of imaging in diagnosis, staging and treatment of neuroblastoma in children. Chinese Journal of Clinical Imaging 2017;28:752-5.
- McHugh K. Renal and adrenal tumours in children. Cancer Imaging 2007;7:41-51. [Crossref] [PubMed]
- Lee JS, Padilla B, DuBois SG, et al. Second malignant neoplasms among children, adolescents and young adults with Wilms tumor. Pediatr Blood Cancer 2015;62:1259-64. [Crossref] [PubMed]
- Tang W, Ren G, Cai R. CT diagnosis of nephroblastoma. Radiology Practice 2019;34:555-9.
- Lee H, Cho JY, Kim SH, et al. Imaging findings of primitive neuroectodermal tumors of the kidney. J Comput Assist Tomogr 2009;33:882-6. [Crossref] [PubMed]
- Zhu MS, Tang WW, Li XH. CT findings of clear cell sarcoma of kidney in children. Journal of Practical Radiology 2016;32:1418-21.
- Huang J, Liu S, Zang YG. CT diagnosis of rare primary renal malignancy in children. Chinese Journal of Medical Imaging 2018;26:197-201.
- Liu C, Cui LG, Wang HL. Renal Ewing's sarcoma/primitive neuroectodermal tumor: a case report and literature review. Beijing Da Xue Xue Bao Yi Xue Ban 2017;49:919-23. [PubMed]
- Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016;70:106-19. [Crossref] [PubMed]


