
Effects of therapeutic hypothermia on the safety of children with severe traumatic brain injury: a systematic review and meta-analysis
Introduction
At present, traumatic brain injury (TBI) can cause disability and death in children and a global major public issue. According to statistics from the Centers for Disease Control and Prevention in the U.S., 52,000 of the 1.7 million cases of TBI die from TBI each year in America. In Europe, the prevalence rate of TBI is 235/0.1 million and the mortality rate reaches 15/0.1 million, while in Asia, the prevalence of TBI is currently unknown (1-3). Statistical data from India shows that about 1.6 million people suffer from TBI every year. In China, TBI is also the main cause of traumatic injury and about 10% of patients with traumatic injury die from TBI. In general, the mortality and incidence of TBI are high worldwide. Following the incidence of TBI, a series of secondary brain injuries, including cerebral ischemia and hypoxia, inflammatory reactions, metabolic dysfunction, and cerebral edema, in addition to the cell injury caused by the trauma itself. Children with TBI usually suffer from severe and persistent neurological and cognitive defects. Their intelligence quotient (IQ) scores are often 18 to 26 points lower than those of normal children. As a result, children with TBI are unable to participate in normal labor and production when they reach adulthood, which results in huge burdens on individuals, families, and society. Relevant statistical studies have revealed that TBI costs about 56 billion dollars each year in the U.S. (4-6).
Therapeutic hypothermia (TH) is a purposeful treatment method for controlling and measuring body temperature and can be used as a neuroprotectant to reduce neuronal loss or injury. The main purpose of TH is to improve the prognosis of patients. According to the achieved body temperature, TH is usually divided into mild (33–36 °C), moderate (28–32 °C), severe (10–28 °C), deep (5–10 °C), and super deep (0–5 °C). In most cases, TH is applied for children with mild (33–36 °C) and moderate (28–32 °C) TBI in clinical treatment, while it is seldom performed on children with severe (10–28 °C), deep (5–10 °C), and super deep (0–5 °C) TBI (7-9). At present, most treatment schemes for children with TBI are formulated based on the treatment schemes of adults with TBI. Currently, there are no definitive and effective therapeutic methods for children with TBI.
However, relevant zoological studies in recent years demonstrated that TH can prevent or reduce secondary injuries through multiple mechanisms in TBI, including reducing brain metabolic need, inflammations, lipid peroxidation, excitotoxicity, and cell death (10,11). In addition, hypothermia treatment for 24 to 48 hours has proven successful in improving prognosis in clinical experiments on TBI and neonatal hypoxic-ischemic encephalopathy (HIE), especially in terms of the reduction in mortality. Despite this success, the effect of TH on children with TBI has remained controversial, with some scholars holding opposing viewpoints to the aforementioned opinions. For instance, the results of some evidence-based medical studies have demonstrated that hypothermia among children with TBI did not exhibit any positive effects on the treatment and prognosis of the disease, and even exacerbated the mortality among these patients. Another example is a study on 96 children with severe TBI, which revealed that hypothermia treatment seemed to be safe, but showed no obvious effect or advantages in terms of patient survival and neurological recovery/improvement (12). There are significant differences in the above research results when analyzing the efficacy of TH in children with TBI, which may be related to the difference in the number of subjects included or the severity of disease in children. Hence, there is a pressing need to investigate the effectiveness of hypothermia treatment more comprehensively on children with TBI (13).
At present, some achievements have been made in animal studies in relevant fields (14). However, TH is still controversial in clinical application in the treatment effect and safety of children with severe TBI. It is difficult to transform these research findings into treatment guidelines. The included subjects in this work were all children with severe brain trauma, and the sample size was increased. A meta-systematic analysis was conducted on mortality, incidence of adverse outcomes, duration of Pediatric Intensive Care Unit (PICU), incidence of infection, and incidence of arrhythmia in the reported subjects, aiming to provide a reference basis to treat the clinically related diseases. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-180/rc).
Methods
Literature retrieval
Literature was screened on PubMed, Web of Science, Embase, Cochrane Library, Nature, NCKI and Wanfang online databases, to retrieve those published during January 2000 and September 2020 using the following search terms: “Chronic kidney disease”, “Cardiovascular disease”, “Medical risk factors”, “Dyslipidemia”, and so on. The search was without language restriction.
How to include and exclude the literature
Relevant articles were included in this research based on the following criteria:
- Domestic and foreign clinical studies on the evaluation of TH in children with severe brain trauma;
- Studies with more than 15 children with severe brain trauma included;
- Articles published during January 2000 and September 2020;
- Clinical randomized controlled trials (RCTs), cohort studies, or case-control studies grouped regarding the treatment;
- Age, gender, and grouping of children were recorded in detail. Meanwhile, indicators such as overall mortality, incidence of adverse outcomes, duration of PICU, incidence of infection, and incidence of arrhythmia in different groups were recorded and statistically analyzed in detail.
Articles were excluded from this research based on the following criteria:
- The same set of data in the article was published repeatedly;
- The article was an overview, conference report, empirical lecture, case report, or comment;
- The study was irrelevant to the research topic;
- The research control group was not set in the article, or the samples between groups were incomparable;
- Articles with unclear reporting on outcome indexes or incomplete result data;
- More than one article was published with the same set of data; in these cases, the article with the highest quality was selected.
Quality evaluation of literatures
Literature quality was evaluated according to the Cochrane system. The two researchers carried out the extraction work separately, and eliminated the literatures failed to satisfy the inclusion criteria and were of low quality. Inconsistent results of the two researchers were discussed or a third party made an evaluation decision.
Bias was evaluated using Cochrane Review Handbook 5.3. The evaluation aspects included the below items: (I) whether the research method was cleaned correctly or not; (II) whether the article explained the cleaning method of random sequence generation clearly; (III) whether the results and data of the article were clear and complete; (IV) whether the article had selective reporting problems, and whether there were deliberate processing problems in the processing of the results; and (V) whether the relevant participants in the experiment had conducted a blind control study. The included literatures were divided into three levels of low, medium, and high deviation. It was preferred to screen the titles of the articles and contact the original authors for articles with missing data. Afterwards, literature analysis was performed by reading abstracts and full texts, and the quality assessment was performed by using the Jadad scale. Finally, a meta-analysis was performed on articles with a score of 3 or more on the Jadad scale. All available information for included studies was finally entered into a Microsoft Excel sheet.
Data retrieval
Two researchers were allowed to read the articles independently to check if they involved case-control or cohort research and if the data were complete in the initial screening. All articles conforming to the inclusion standards were evaluated. The unusable articles with duplicate reports, poor quality, and insufficient report information were excluded. Data were extracted to set up the database and check the information according to the established tables. For reports with incomplete data, the authors were contacted to determine and exclude unusable articles. Inconsistent views between the 2 researchers were addressed by discussion with a third party. The data was retrieved after the whole text was obtained. Among the repeated articles, the one with the latest data was selected. The extracted data included the title, first author, year of publication, author information, and source of the article; the gender, age, research sample size, and baseline comparability of the subjects, the research methods utilized in the study, the research scheme design, the intervention measures of patients in different groups, the outcome evaluation indexes, and the outcome data.
Data extraction
A forest plot was constructed to clearly display the research results of every individual article and combine articles with corresponding CIs. If there was no overlap between the CIs of each research result, statistical inhomogeneity was deemed to exist. With the acceptable inhomogeneity, random models were combined with fixed models for further subgroup analysis. According to different designs, studies with acceptable inhomogeneity were divided into subgroups.
Next, the different study designs were investigated to determine the influencing size of each sub-group. When the inhomogeneity could not be ignored to deal with inhomogeneity without processing different study designs, these designs could be ignored.
Statistical analysis
Sensitivity was analyzed by analyzing whether individual articles affected the overall results. Each article was sequentially removed. It was believed that the comprehensive studies were affected in two cases in the research. The putative value of the combined effect size was more than 95% of the combined effect size. When an article was deleted, the results became significantly different. Slight differences in the influences of an article on the overall results indicated the sensitivity of the combination results with unstable outcomes. However, the results showed a stable sensitivity and correct conclusion.
RevMan 5.3 software from the Northern Europe Cochrane collaboration network was used for data statistics and analysis. Firstly, the chi-square test was for the heterogeneity of literatures, and the significance level was set as α=0.05 and P<0.05. Also, the Peto method was adopted to analyze the heterogeneity of the included articles. I2<50% revealed that there was no heterogeneity, and in these cases, the articles were analyzed using fixed-effects models. Meanwhile, I2>50% demonstrated that there was heterogeneity between the included studies, and the articles were analyzed using random-effects models. The results of measurement data adopting the same measurement unit were expressed by the mean difference (MD). The results of enumeration data were expressed by the odds ratio (OR) or relative risk (RR). Furthermore, all of the results were denoted by the 95% CI. Funnel plots were drawn, and the publication bias of articles was assessed by the symmetry of the funnel plots and the concentration of studies towards the middle line. Sensitivity analysis was utilized to assess the reliability and stability of the results. P<0.05 represents the statistically significant result of comparison.
Results
Literature retrieval and profile analysis
A total of 3,812 relevant articles were initially retrieved and 2,506 relevant articles were retrieved from the databases. In addition, 1,306 articles were retrieved and obtained from the registers. After the titles were read, 1,581 unqualified articles were excluded, and 2,231 articles were included for subsequent analysis. After the abstracts were read, 1,702 unqualified articles were excluded. Next, the abstracts of the retrieved articles were read carefully, and 518 unqualified articles were excluded. Thereafter, the full texts of the 11 remaining articles were downloaded. After further studying the full texts, only five articles that satisfied the requirements were included (15-19). Figure 1 displays the literature retrieval process. The included articles were revealed in Table 1.
Table 1
Bias risk evaluation
The Cochrane Handbook 5.3 was to assess the bias risk of the five included articles, and the results were displayed in Figures 2,3. The diagrams were expressed by Review Manager 5.3 software. According to the analysis of Figures 2,3, all of the items of one included article were at low risk, one item among three articles was at a high risk of bias, and the bias risk of one item in one article was unknown.
Overall mortality analysis
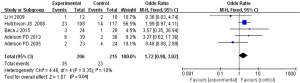
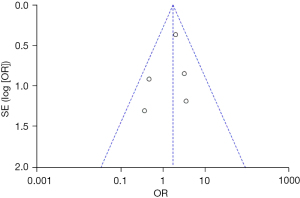
Based on the investigation and screening of articles, the effect of TH on the mortality of children with severe TBI was compared and researched in five articles. The results of heterogeneity analysis were I2=10% and P=0.35, which indicated no obvious heterogeneity. Hence, fixed-effects models were for meta-analysis. The structural analysis model showed that OR =1.72, 95% CI: 0.98–3.02, Z=1.87, and P=0.04, which demonstrated that there were obvious differences in the mortality of children with TBI between the hypothermia and normal temperature therapy groups (P<0.05), as shown in Figure 4. Figure 5 displayed the results of the publication bias analysis. The funnel plot was generally symmetrical and most data were on both sides of the central axis, which indicated no obvious publication bias.
Adverse outcome rate analysis
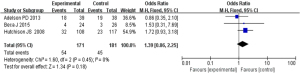
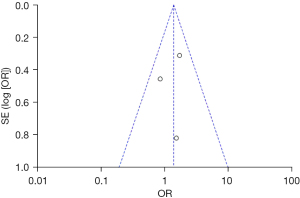
Based on the investigation and screening of articles, the effect of TH on the adverse outcomes of children with severe TBI was compared and researched in three articles. The results of the heterogeneity test were I2=0% and P=0.45, showing no obvious heterogeneity. Hence, fixed-effects models were selected for meta-analysis. The structural analysis models demonstrated that OR =1.39, 95% CI: 0.86–2.25, Z=1.34, and P=0.18. Figure 6 suggested that there was no obvious difference in the adverse outcomes of children with severe TBI between the hypothermia and normal temperature therapy groups (P>0.05). Figure 7 demonstrated the publication bias analysis results of the included articles. The funnel plot was generally symmetrical and all article data were within the plot and on both sides of the central axis, showing no obvious publication bias.
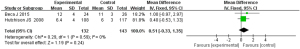
Comparison of the duration of intensive care for children with severe TBI
Based on the investigation and screening of articles, the effect of TH on the duration of intensive care for children with severe TBI was compared and researched in two articles. The results of heterogeneity analysis were I2=0% and P=0.59, which revealed that the heterogeneity was not obvious. Therefore, fixed-effects models were selected for meta-analysis. The structural analysis models showed that MD =0.51, 95% CI: −0.33 to 1.35, Z=1.19, and P=0.24. No obvious difference was demonstrated in the duration of intensive care for children with severe TBI between the hypothermia and normal temperature therapy groups (P>0.05) (Figure 8). Figure 9 presents the publication bias analysis results. According to Figure 9, the article data were all within the plot and on both sides of the central axis, which demonstrated that the publication bias was not great.
Comparison of incidence of infection
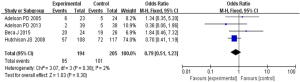
Based on the investigation and screening of articles, the effect of TH on the incidence of infection among children with severe TBI was compared and researched in four articles. The results of heterogeneity analysis were I2=2% and P=0.38, which revealed no obvious heterogeneity. Therefore, fixed-effects models were selected for meta-analysis. The structural analysis models demonstrated that OR =0.79, 95% CI: 0.51–1.23, Z=1.03, and P=0.30. The incidence of infection among children with severe TBI between the hypothermia and normal temperature therapy groups was not obvious (P>0.05), as demonstrated in Figure 10. Figure 11 displays the publication bias analysis results of the included articles. The funnel plot was generally symmetrical and the data of the included articles were all within the funnel plot, indicating no obvious publication bias.
Comparison of incidence of arrhythmia
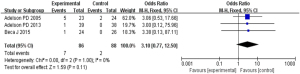
Based on the investigation and screening of articles, the effect of TH on the incidence of arrhythmia was compared and researched in three articles. The results of heterogeneity analysis were I2=0% and P=1.00, which indicated no obvious heterogeneity. Hence, fixed-effects models were selected for meta-analysis. The structural analysis models demonstrated that OR =3.10, 95% CI: 0.77–12.50, Z=1.59, and P=0.11. No obvious difference in the incidence of arrhythmia among children with severe TBI was found between the hypothermia and normal temperature therapy groups (P>0.05), as illustrated in Figure 12. Figure 13 displays the publication bias analysis. The funnel plot was generally symmetrical and the data of the included articles were all within the funnel plot, which revealed that no obvious publication bias was found.
Discussion
TBI is a very complex and severe injury, which may be caused by direct external force or various cascade reactions resulting from primary injuries. Consequently, secondary injuries occur. At present, TBI can result in death and dysfunction in children. Following the incidence of TBI, a series of secondary brain injuries, including cerebral ischemia and hypoxia, inflammatory reactions, metabolic dysfunction, and cerebral edema, in addition to the cell injury caused by the trauma itself (20). The series of complications and sequelae triggered by TBI is the focus and challenge of treatment. Compared with that of adults, the structure of children’s brains is relatively special. In general, cerebral edema and intracranial hypertension are more significant among children than adults following TBI. In addition, the sequelae caused by secondary brain injuries, such as brain paralysis and traumatic epilepsy, are more common among children than among adults. As a result, the treatment of brain injuries among children is more complex and difficult than in adults. In most cases, the prognosis of the treatment of children is relatively poorer (21,22).
Numerous recent studies have shown that the clinically appropriate reduction in body temperature could avoid the death of neurons, which implies that the reduction in temperature might protect neurons (23-25). Furthermore, recent relevant studies have also demonstrated that hypothermia plays a neuroprotective role both in cardiac arrest and cerebral hypoxic and ischemic injuries caused by neonatal suffocation. In addition, animal experiments have confirmed that hypothermia also protects against transient cerebral ischemia, cerebral trauma, and spinal cord injury. At times, hypothermia has been known as “thanatosis”. Important metabolic processes in the body are slowed down or stopped, which avoids death. TH is a purposeful treatment method for controlling and measuring body temperature and can be used as a neuroprotectant to reduce neuronal loss or injury. The main purpose of TH is to improve the prognosis of patients. TH has been applied in clinical auxiliary treatment since being re-valued by scholars in the 1990s (26-28). Many studies have suggested that TH could improve the neurological prognosis and reduce adverse reactions in children with TBI. At present, the mechanism of action of this method is not very clear. Current studies have demonstrated that the main effects of TH are as follows:
- TH could reduce the metabolism of cerebral cells;
- TH could reduce the accumulation of massive amounts of cytotoxin;
- TH could resist cell apoptosis;
- TH could protect the blood-brain barrier.
Also, relevant animal experiments revealed that there were no abnormal pathological changes in the hearts, lungs, spleens, kidneys, and small intestines of newborn pigs after TH. Moreover, some scholars performed hypothermia on 11 neonates with ischemic TBI and found no abnormalities in liver, kidney, or coagulation functions (29). The above studies have highlighted the safety of TH application in children with TBI diseases. However, there are multiple disputes on the efficacy of TH at present. For example, some scholars randomly divided 148 children with TBI into TH and normal temperature treatment groups at a ratio of 2:1. After a 6-month of follow-up visits and treatment, no significant difference was found in the Glasgow outcome scale (GOS) scores and mortality between the two groups of patients (29,30). Thus, this treatment method requires further research.
Therefore, articles related to the effect of TH in children with TBI were retrieved. We also conducted a comprehensive and systematic analysis in terms of mortality, the incidence of adverse outcomes, the duration in PICU, the incidence of infection, and the incidence of arrhythmia. Our results indicated that all indexes of the TH and normal temperature treatment groups showed no remarkable difference, which suggested that TH might not provide a positive therapeutic effect in children with TBI. However, our results might have been affected by deviations resulting from differences in the types of injuries among children and follow-up times in the included articles, which were also limitations of this research. Therefore, more relevant high-quality clinical studies are needed to support evidence-based medical research and analysis.
Conclusions
Articles related to the influences of TH on children with TBI were retrieved, and a comprehensive and systematic analysis was carried out in terms of mortality, the incidence of adverse outcomes, the duration of stay in PICU, the incidence of infection, and the incidence of arrhythmia. We found no significant differences in any of these indexes between the TH and normal temperature treatment groups, which implied that TH might not provide positive therapeutic effects in children with TBI. Another limitation of this study was that neuron-specific enolase (NSE) and other biological indexes were not included. In future studies, more high-quality clinical articles will be included to improve the results and continue the research into this issue.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-180/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-180/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Klein S. Traumatic Brain Injury in Children. J Binocul Vis Ocul Motil 2020;70:115. [Crossref] [PubMed]
- Reith W, Kettner M, Yilmaz U. Nonaccidental traumatic brain injury in infants and children. Radiologe 2021;61:742-7. [Crossref] [PubMed]
- Cooper DJ, Nichol AD, Bailey M, et al. Effect of Early Sustained Prophylactic Hypothermia on Neurologic Outcomes Among Patients With Severe Traumatic Brain Injury: The POLAR Randomized Clinical Trial. JAMA 2018;320:2211-20. [Crossref] [PubMed]
- Koenig MA. Cerebral Edema and Elevated Intracranial Pressure. Continuum (Minneap Minn) 2018;24:1588-602. [Crossref] [PubMed]
- Quine EJ, Murray L, Trapani T, et al. Thromboelastography to Assess Coagulopathy in Traumatic Brain Injury Patients Undergoing Therapeutic Hypothermia. Ther Hypothermia Temp Manag 2021;11:53-7. [Crossref] [PubMed]
- Docherty A, Emelifeonwu J, Andrews PJD. Hypothermia After Traumatic Brain Injury. JAMA 2018;320:2204-6. [Crossref] [PubMed]
- Schizodimos T, Soulountsi V, Iasonidou C, et al. An overview of management of intracranial hypertension in the intensive care unit. J Anesth 2020;34:741-57. [Crossref] [PubMed]
- Escamilla-Ocañas CE, Albores-Ibarra N. Current status and outlook for the management of intracranial hypertension after traumatic brain injury: decompressive craniectomy, therapeutic hypothermia, and barbiturates. Neurologia (Engl Ed) 2020. [Epub ahead of print].
- Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev 2019;12:CD003983. [Crossref] [PubMed]
- Bouzat P, Payen JF. Therapeutic hypothermia after traumatic brain injury: Wrong hypotheses may lead to specious interpretations. Anaesth Crit Care Pain Med 2019;38:95-6. [Crossref] [PubMed]
- Lateef S, Holman A, Carpenter J, et al. Can Therapeutic Hypothermia Diminish the Impact of Traumatic Brain Injury in Drosophila melanogaster? J Exp Neurosci 2019;13:1179069518824852. [Crossref] [PubMed]
- Chen H, Wu F, Yang P, et al. A meta-analysis of the effects of therapeutic hypothermia in adult patients with traumatic brain injury. Crit Care 2019;23:396. [Crossref] [PubMed]
- Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Pediatr Crit Care Med 2019;20:280-9.
- Kalisvaart ACJ, Prokop BJ, Colbourne F. Hypothermia: Impact on plasticity following brain injury. Brain Circ 2019;5:169-78. [Crossref] [PubMed]
- Beca J, McSharry B, Erickson S, et al. Hypothermia for Traumatic Brain Injury in Children-A Phase II Randomized Controlled Trial. Crit Care Med 2015;43:1458-66. [Crossref] [PubMed]
- Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med 2008;358:2447-56. [Crossref] [PubMed]
- Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol 2013;12:546-53. [Crossref] [PubMed]
- Li H, Lu G, Shi W, et al. Protective effect of moderate hypothermia on severe traumatic brain injury in children. J Neurotrauma 2009;26:1905-9. [Crossref] [PubMed]
- Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery 2005;56:740-54; discussion 740-54. [Crossref] [PubMed]
- Carlisle MA, Bennett TD. Phenotyping in Pediatric Traumatic Brain Injury. Pediatr Crit Care Med 2018;19:998-9. [Crossref] [PubMed]
- Dewan S, Schimmel S, Borlongan CV. Treating childhood traumatic brain injury with autologous stem cell therapy. Expert Opin Biol Ther 2018;18:515-24. [Crossref] [PubMed]
- Bernstein JE, Ghanchi H, Kashyap S, et al. Pentobarbital Coma With Therapeutic Hypothermia for Treatment of Refractory Intracranial Hypertension in Traumatic Brain Injury Patients: A Single Institution Experience. Cureus 2020;12:e10591. [Crossref] [PubMed]
- Vermeersch V, Huet O. Prophylactic hypothermia for traumatic brain injury patients: It is not cool to be cooled. Anaesth Crit Care Pain Med 2019;38:97-8. [Crossref] [PubMed]
- Engrand N, Pharaboz A, Dinkelacker V. Prophylactic Hypothermia for Severe Traumatic Brain Injury. JAMA 2019;321:1725. [Crossref] [PubMed]
- Cooper DJ, Nichol AD, McArthur C. Prophylactic Hypothermia for Severe Traumatic Brain Injury-Reply. JAMA 2019;321:1726. [Crossref] [PubMed]
- Zhu L. Hypothermia Used in Medical Applications for Brain and Spinal Cord Injury Patients. Adv Exp Med Biol 2018;1097:295-319. [Crossref] [PubMed]
- Olah E, Poto L, Hegyi P, et al. Therapeutic Whole-Body Hypothermia Reduces Death in Severe Traumatic Brain Injury if the Cooling Index Is Sufficiently High: Meta-Analyses of the Effect of Single Cooling Parameters and Their Integrated Measure. J Neurotrauma 2018;35:2407-17. [Crossref] [PubMed]
- Watson HI, Shepherd AA, Rhodes JKJ, et al. Revisited: A Systematic Review of Therapeutic Hypothermia for Adult Patients Following Traumatic Brain Injury. Crit Care Med 2018;46:972-9. [Crossref] [PubMed]
- Wu X, Tao Y, Marsons L, et al. The effectiveness of early prophylactic hypothermia in adult patients with traumatic brain injury: A systematic review and meta-analysis. Aust Crit Care 2021;34:83-91. [Crossref] [PubMed]
- Olah E, Poto L, Rumbus Z, et al. POLAR Study Revisited: Therapeutic Hypothermia in Severe Brain Trauma Should Not Be Abandoned. J Neurotrauma 2021;38:2772-6. [Crossref] [PubMed]






