
Inherited and acquired causes renal vein thrombosis in an 11-year-old boy who was relieved after piperacillin-tazobactam and rivaroxaban treatment: a case report
Introduction
Physiological hypercoagulability is a well-known condition in older populations compared with children and neonates. Renal vein thrombosis (RVT), the presence of thrombus in the major renal veins or its tributaries, is a rare clinical entity for all populations. It can present acutely or go unnoticed, resulting in acute kidney injury or chronic kidney disease (1). There are no specific numbers available for the frequency of RVT by age. However, venous thromboembolism (VTE) is far less common in children and neonates than adults, although its frequency is increasing (2). Here, we report on an 11-year-old Chinese boy who presented with low back discomfort, diagnosed with spontaneous bilateral RVT and inferior vena cava thrombosis. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-95/rc).
Case presentation
An 11-year-old Chinese boy attended his local hospital with mycoplasma pneumoniae pneumonia (MPP) on January 13, 2022 (Table 1). Three days previously, he had felt low back discomfort and was afraid to walk. The Doppler ultrasound showed an increased echo and decreased blood flow signal in the upper polar region of the right renal parenchyma. The computed tomography (CT) showed bilateral RVT, right renal swelling, and inferior cava thrombosis. He was transferred to our hospital for further treatment. He continued to experience flank pain and was unable to walk freely.
Table 1
| Time | The treatment process |
|---|---|
| January 13, 2022 | The boy attended his hospital with MPP, and was transferred to our hospital for further treatment. The patient was diagnosed with thrombosis of the bilateral renal veins and inferior cava, pulmonary embolism, and MPP. He was treated with rivaroxaban (10 mg twice per day), urokinase for thrombosis, and azithromycin for MPP |
| January 14, 2022 | The patient’s symptoms improved. Therefore, the dosage of rivaroxaban was decreased from 10 to 5 mg twice per day |
| January 15, 2022 | The patient developed a fever of 39.2 ℃ and complained of right chest pain. All the above imaging suggested that antithrombotic therapy was insufficient |
| January 16, 2022–January 30, 2022 | The imaging results and clinical features, supported the diagnosis of severe refractory MPP with azithromycin resistance. Therefore, azithromycin was swapped for piperacillin-tazobactam, and the rivaroxaban dosage was increased from 5 to 10 mg twice per day. Following a medication regimen of piperacillin-tazobactam for 1 week and rivaroxaban (10 mg, twice daily), he was discharged |
| January 31, 2022–April 30, 2022 | There is no thrombosis and other side effects or complications occurred in the following 3 months |
MPP, mycoplasma pneumoniae pneumonia.
On admission, his temperature was 36.1 ℃, with a pulse of 126 beats per minute, respiratory rate of 20 breaths per minute, and blood pressure of 111/72 mmHg. Other systemic examinations were unremarkable, including a normal drum sound on abdominal percussion.
Laboratory examinations showed a normal complete blood count with an elevated neutrophil percentage (73%) (reference range, 31–70%). A routine urine test showed an elevated red blood cell (RBC) count (234 cells/µL) (reference range, 0–17 cells/µL) and positive protein, occult blood, and ketone body. His biochemistry profile showed elevated levels of serum total cholesterol (TC) (6.57 mmol/L) (reference range, 2.80–5.17 mmol/L), low-density lipoprotein cholesterol (LDL-C) (4.05 mmol/L) (reference range, 0–3.20 mmol/L), lactate dehydrogenase (LDH) (562 U/L) (reference range, 114–256 U/L), and α-hydroxybutyrate dehydrogenase (HBDH) (455 U/L) (reference range, 72–182 U/L). Serum inflammation markers showed higher C-reactive protein (CRP) (145 mg/L) (reference range, 0–10 mg/L), interleukin-6 (IL-6) (72.55 pg/mL) (reference range, 0–7.00 pg/mL), and IL-8 (40.72 pg/mL) (reference range, 0–20.60 pg/mL). The coagulation profile showed decreased levels of fibrinogen (1.88 g/L) (reference range, 2.38–4.98 g/L), plasminogen activity (52.0%) (reference range, 80–130%), antithrombin (AT) III activity (29%) (reference range, 83–128%) (Figure 1), protein C (PC) activity (55%) (reference range, 70–140%), and protein S (PS) activity (55.0%) (reference range, 63.5–149.0%). There were elevated levels of thrombin-antithrombin complexes (TAT) (49.3 ng/mL) (reference range, 0–4.00 ng/mL), plasmin-α2 plasmin inhibitor complex (PIC) (15.075 ng/mL) (reference range, 0–0.800 ng/mL), and D-dimer (5.2 mg/L) (reference range, 0–0.55 mg/mL). The other coagulation tests were generally within the reference ranges, including thrombomodulin (TM) and tissue plasminogen activator/plasminogen activator inhibitor-1 (t-PAIC). Serum compliment (C) examinations were C1q 245 mg/L (reference range, 159–233 mg/L), C3 1.52 g/L (reference range, 0.90–1.80 g/L), C4 0.40 g/L (reference range, 0.90–1.80 g/L). Tumor biomarkers showed higher carbohydrate antigen (CA 19-9) 56.30 U/mL (reference range, 0–35.00 U/mL) and neuron specific enolase (7.48 ng/mL) (reference range, 0–6.00 ng/mL). Mycoplasma pneumoniae (MP) IgM/IgG antibodies and RNA were negative. Hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) antibodies were negative. All other autoantibodies were negative, including anticardiolipin antibody, anti-β2-gp1 antibody, and lupus anticoagulant.
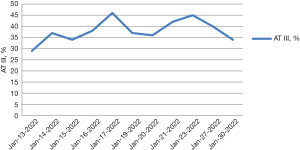
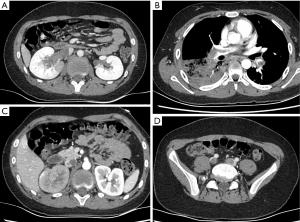
The patient was diagnosed with thrombosis of the bilateral renal veins and inferior cava, pulmonary embolism, and MPP. He was treated with rivaroxaban (10 mg twice per day), urokinase for thrombosis, and azithromycin for MPP. On day 2 after admission, his symptoms improved. Therefore, the dosage of rivaroxaban was decreased from 10 to 5 mg twice per day. On day 3 after admission, the patient developed a fever of 39.2 ℃ and complained of right chest pain. Enhanced CT revealed new thromboses in the bilateral pulmonary trunks and arteries, inferior cava, right renal veins, bilateral common iliac veins, and internal iliac vein (Figure 2). The ultrasonography showed a strip hypoecho at the pulmonary artery bifurcation. All the above imaging suggested that antithrombotic therapy was insufficient.
We questioned whether MPP was involved in the thrombosis. Serum ferritin result revealed a far higher level (442.3 ng/mL) than the reference range of 23.9–336.2 ng/mL. The laboratory results of ferritin >328 ng/mL, neutrophil percentage >70%, LDH >478 U/L, and CRP >110 mg/L, together with the imaging results and clinical features, supported the diagnosis of severe refractory MPP with azithromycin resistance. Therefore, azithromycin was swapped for piperacillin-tazobactam, and the rivaroxaban dosage was increased from 5 to 10 mg twice per day. His symptoms gradually returned to normal and so did the coagulation biomarkers, except AT III activity. The AT III activity remained low. The family history was re-examined and revealed that both his father and grandfather had experienced spontaneous pulmonary thrombosis around the age of 30. We concluded that the thrombogenesis arose from primary AT III deficiency and severe refractory MPP. Following a medication regimen of piperacillin-tazobactam for 1 week and rivaroxaban (10 mg, twice daily), he was discharged on January 30, 2022 and no thrombosis and other side effects or complications occurred in the next 3 months.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent for publication was obtained from the patient’s legal guardian because he was under 18 years old. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The incidence of VTE in children is very low, estimated at 0.07–0.49 per 10,000 children (3). However, in hospitalized children, the rate is increased 100 to 1,000 folds, to ≥58 per 10,000 admissions (4).
In children 11–18 years old, thrombosis accounts for up to 50% of pediatric VTE. Renal vein VTE comprises a significant proportion of events in neonates but is exceedingly rare in older children where lower extremity VTE is more likely. Although rare, non-extremity VTE in children may arise in the portal, splenic, mesenteric, and pulmonary vessels, or the cerebral sinuses (5). Our case was rare and involved thromboses in the renal veins, inferior vena cava, and pulmonary vessels.
The cause of thrombosis is multifactorial. As noted, an imbalance in endogenous anticoagulation and hemostasis is the pathophysiological mechanism for thrombosis. Historically, three common factors predispose to thrombosis: vascular endothelial injury, hypercoagulability, and arterial or venous blood stasis. In the present case, the normal TM and t-PAIC, reflecting the integrity of the endothelial cells, suggested a normal endothelial lining of the vessel wall. Moreover, the patient was an 11-year-old boy with no obvious blood stasis history, and he was being treated for MPP when thrombosis occurred. What led to his hypercoagulable state and thrombosis? Both acquired and inherited factors were considered. We decided that the patient’s thrombosis was related to heredity because both the boy’s father and grandfather experienced spontaneous pulmonary thrombosis around the age of 30. And during hospitalization, the patient’s plasma AT III activity was consistently lower than 50%, while all other coagulation markers returned to normal before discharge. The negative anticardiolipin antibody, anti-β2-gp1 antibody, and lupus anticoagulant findings precluded the possibility of antiphospholipid syndrome. So, we concluded that he had an inherited AT III deficiency. Additionally, we diagnosed acquired thrombosis because he had severe refractory MPP with macrolide (azithromycin) resistance. A recent Chinese study confirmed that refractory MPP frequently presents with necrotizing pneumonia, airway occlusion, or thrombosis (6).
There are no anticoagulant drugs approved for pediatric use, and there is a lack of specific research trials in children. Much of the evidence for treatment is inferred from adult practice, despite the significant differences between adults and children in the epidemiology and pathophysiology of thrombosis, the physiology of the coagulation system, and the impact of this on the pharmacology of antithrombotic agents (7). In our case, as a result of inherited thrombosis with low plasma AT III activity and acquired thrombosis from refractory MPP, oral rivaroxaban at a dosage of 10 mg twice daily was the final medical decision. For this patient, the dosage of 5 mg twice daily was inappropriate and resulted in the reoccurrence of thrombosis.
An abnormally higher CA 19-9 level of 56.30 U/mL was found in the present case. As one of several tumor markers, it has been suggested that CA 19-9 may trigger thrombotic events in cancer patients (8). However, there was no imaging data to support a tumor diagnosis in our case. And no other CA 19-9 levels were taken during the patient’s hospitalization. Therefore, whether CA 19-9 plays a role in all patients with thrombosis needs further observation and research.
Conclusions
This study presents a rare case of a teenager with inherited and acquired hypercoagulability. For refractory MPP pediatric patients with thrombosis, clinicians should consider whether hereditary factors, such as AT III deficiency, are involved in the thrombosis.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Hebei Province (No. H202120603), the Science and Technology Research Programme of the Health Commission of Hebei Province (No. 20201185) and the Key Research and Development Projects of Hebei Province (No. 182777181).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-95/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-95/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent for publication was obtained from the patient’s legal guardian because he was under 18 years old. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mazhar HR, Aeddula NR. Renal Vein Thrombosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing, June 25, 2021.
- Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program 2018;2018:399-404. [Crossref] [PubMed]
- Mahajerin A, Croteau SE. Epidemiology and Risk Assessment of Pediatric Venous Thromboembolism. Front Pediatr 2017;5:68. [Crossref] [PubMed]
- Raffini L, Huang YS, Witmer C, et al. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics 2009;124:1001-8. [Crossref] [PubMed]
- Pergantou H, Avgeri M, Komitopoulou A, et al. Venous thromboembolism at uncommon sites in neonates and children. J Pediatr Hematol Oncol 2014;36:624-9. [Crossref] [PubMed]
- Liu J, He R, Wu R, et al. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing Children's hospital. BMC Infect Dis 2020;20:51. [Crossref] [PubMed]
- Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e737S-801S.
- Awkar N, Amireh S, Rai S, et al. Association between Level of Tumor Markers and Development of VTE in Patients with Pancreatic, Colorectal and Ovarian Ca: Retrospective Case- Control Study in Two Community Hospitals. Pathol Oncol Res 2018;24:283-7. [Crossref] [PubMed]


