
Predicting the probability of a live birth after a freeze-all based in vitro fertilization-embryo transfer (IVF-ET) treatment strategy
Introduction
The first live birth following embryo cryopreservation was reported by a Dutch team in 1984. Since then, this technique has become widespread throughout the world and led to rapid development of in vitro fertilization-embryo transfer (IVF-ET)/intracytoplasmic sperm injection (ICSI) protocols (1). Currently, the quality of frozen embryos and their potential for implantation are similar to or even exceed those of fresh embryos (2,3). This favors the so-called ‘freeze-all’ strategy performed with the elective cryopreservation of all viable embryos in a fresh IVF/ICSI cycle with the frozen-thawed embryos transferred in the following cycles (4). In order to place the embryos in a more favorable intrauterine environment (and thereby optimize IVF/ICSI outcome), without facing the possible negative effect of ovarian hyperstimulation on the endometrium, the metric for success of IVF/ICSI has changed from live-birth rate in a single fresh cycle (5) to cumulative live birth rates (CLBRs). In practice, the freeze-all strategy can reduce the risk of ovarian hyperstimulation syndrome (OHSS) in the ovarian stimulation cycle by avoiding a pregnancy (6) and obtain better results. Previous studies have shown improved live birth rate (LBR) and a significant decrease in the risk of OHSS and adverse perinatal outcomes in pregnancies after the transfer of frozen embryos (3,7,8). Chen et al. (9) report the results of a multicenter, randomized clinical trial among infertile women with polycystic ovary syndrome (PCOS). As expected, the rate of live birth was significantly higher (49.3% vs. 42.0%) and the rate of the OHSS significantly lower in the frozen-embryo group than in the fresh embryo group (1.3% vs. 7.1%). Though other studies, including two randomized trials showed a similar LBR comparing freeze-all strategy with fresh embryo transfer in general infertile patients, a lower risk of OHSS in the frozen-thawed embryo transfer (FET) group was found (10,11). Thus, among generally infertile patients, including the subgroups of patients who are at high risk for OHSS or those with improper endometrial status, a freeze-all strategy may be beneficial.
The CLBR estimates the outcome of the entire course of treatment to provide an all-inclusive success rate (12,13). However, CLBRs are often reported either as one overall rate which include fresh and FET cycles per initiated ovarian stimulation (14) or the likelihood of a live birth during repeat IVF/ICSI cycles which include frozen embryo replacements as well as subsequent treatment episodes (15,16). However, these studies were performed in patients with fresh IVF/ICSI treatments. As previous studies support the adoption of an individualized “freeze-all” strategy instead of fresh ET to get better IVF outcomes (17,18), the shift from fresh ET to FET may become a trend in many programs. Therefore patients and physicians are keen to know the chance of a live birth for the individual couple over an entire IVF/ICSI program combined with a “freeze-all” strategy. A retrospective observational study found variables of freeze-all-IVF cycles with single blastocyst FET selected by multiple logistic regression to predict LB significantly were female age, infertility duration, FET number and blastocyst quality (19). They also found ovarian reserve variables were not significantly selected by the regression model in predicting LBR. However, in this study only the first oocyte retrieval attempt performed for each patient-couple were retained and all IVF treatments were performed using single blastocyst FET. Its design may limit the generalizability of evidence.
The objectives in the present study are to explore the predictors of CLBRs following one complete IVF cycle for unselected patients using a “freeze-all” strategy, and then to develop and validate a prediction model based on patient demographic and cycle characteristics. The model is designed to estimate the individualized CLBR after all FET cycles from one stimulated cycle. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-589/rc).
Methods
Patients
We performed a retrospective study with linkage of cycles to individual patients and data on birth outcomes at the Department of Assisted Reproduction of Shanghai First Maternity and Infant Hospital of Tongji University School of Medicine. In this population-based cohort study, records of all complete IVF/ICSI cycles were defined as all attempts at FET resulting from one episode of ovarian stimulation. Our cohort was limited to ovarian stimulation cycles initiated between January 1, 2015 and December 30, 2020, with live-birth outcome data collected up to December 2021. Patients were followed by face to face and/or telephone conversations during treatment at our department for at least 1 year until either termination of treatment or delivery of a first live birth. All women undergoing IVF/ICSI treatment with the freeze-all strategy were screened. Exclusion criteria were uterine abnormality, donor insemination, “Re-entries” of couples after a live birth in a preceding cycle, and those patients who were not undergoing the first ovarian stimulation in our center. After exclusions were mode, our population of 7,602 women undergoing 9,964 FET cycles were analyzed (Figure 1).
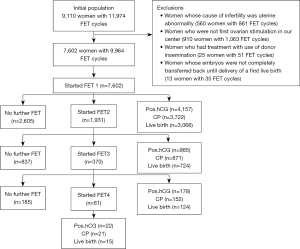
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Shanghai First Maternity and Infant Hospital of Tongji University School of Medicine (No. KS21282) and individual informed consent for this retrospective study was waived.
FET
Patients underwent regimens for ovarian stimulation, monitoring, and oocyte collection as previously described (20-22). In general, fertilization was carried out in vitro, by either conventional IVF or ICSI, depending on semen parameters. Embryos were cultured and scored (23) as previously described. Embryos (non-top-quality) not suitable for cryopreservation on day 3 were cultured to day 5 or 6 and vitrified if they reached the blastocysts stage. Then, good-morphology blastocysts were vitrified on day 5 or 6. The vitrification procedure was performed using the Cryotop carrier system (Kitazato Biopharma Co., Tokyo, Japan).
IVF/ICSI cycles with the use of cryopreserved embryos were performed either in natural cycles for women with regular menses, or in induced ovulation cycles for women with anovulatory infertility; or in case of thin endometria, in hormonal substitution cycles (22). Once pregnancy was achieved, exogenous progesterone (P) supplemention was continued until 10 weeks of gestation.
Reproductive outcomes
Live birth was defined as the complete expulsion or extraction from its mother as product of fertilization, irrespective of the duration of the pregnancy, which, after such separation, breathes or shows any other evidence of life such as heart beat, umbilical cord pulsation, or definite movement of voluntary muscles, irrespective of whether the umbilical cord had been cut or the placenta was attached (24). CLBR was defined as the total chance of a live birth with all cycles up to and including that given cycle number. Thus, CLBR was calculated by including the first live birth generated during the complete IVF cycle as the numerator and censoring additional live births out. The denominator was defined as all women allocated to treatment (12). The conservative CLBRs were based on the assumption that none of the women who discontinued treatment would have had a live birth. These two curves show the best- and worst-case estimates of the CLBRs in the study group. The optimal CLBRs were based on the assumption that women who discontinued treatment would have had the same chance of a pregnancy resulting in a live birth as those who remained in treatment. These CLBRs reflect the worst- and best-case estimates, respectively.
Statistical analysis
Descriptive statistics were calculated for patients and treatment characteristics at the IVF/ICSI-FET treatment. Data were presented as mean ± standard deviation (SD) if they demonstrated normal distributions, or presented as median (interquartile range) for non-normal distributions, and qualitative data were presented as percentages. Normality was tested using the Kolmogorov-Smirnov test. The STROBE reporting checklist was completed.
Model development
A discrete time logistic regression model (25) was used to predict the chance of a live birth after a complete IVF/ICSI-FET cycle using the characteristics of patients and cycle characters. From this model, we treated the FET cycle number as a discrete time variable and calculated the cumulative probability of a live birth over sequential complete FET cycles up to cycle number 4.
First, univariate models were fitted with the LBR as the dependent variable for all individual predictors (adjusted for complete cycle number). The duration of infertility, number of previous IVF failures, number of frozen embryos in cleavage stage, number of frozen embryos in blastocyst stage and number of blastocyst transferred all had non-linear relations with the LBR so they were analyzed with restricted cubic splines. To prevent overfitting of the model, the data was randomly divided into two parts: 70% of all observations were used for the primary analyses (training set; n=7,007) and 30% of observations were used for internal model validation (validation set; n=2,957). Variables with P<0.05 in univariate models were selected for further analysis of the final multivariate discrete time logistic regression model by means of a manual backward selection process. For the final multivariate model, multicollinearity among all variables was examined. Effect estimates were presented with the odds ratio (OR), 95% confidence interval (CI), P value and adequacy for each predictor. All analyses were performed in statistical software R version 3.4.2 (R Core Team, Vienna, Austria). A two-sided P value <0.05 was considered to be statistically significant.
Missing data
This procedure assumes that the missing data are “missing at random”. We compared the characteristics of the women with or without missing data. Single imputation was performed for the predictors with missing data.
Predictive ability
We used c-statistics and calibration to assess the ability of the multivariate models. The c-statistic is routinely used in the medical literature to quantify the capacity of the estimated risk score in discriminating subjects with different event times and is analogous to the area under the receiver-operating characteristic curve in assessing the model’s discriminative capacity (26-28). Calibration, assessed by whether predicted probabilities are consistent with observed proportions, was quantified by means of the Hosmer-Lemeshow χ2 statistic (26,27). Moreover, adequacy statistics were calculated to investigate the explanatory value of each predictor to the entire set (29).
Results
Characteristics of the patients
After exclusions (see methods), the eligible dataset included 7,602 women who underwent 9,964 FET cycles (see Figure 1).
Baseline characteristics of the cohort and clinical characteristics of their oocyte retrieval cycle resulting from one episode of ovarian stimulation are summarized in Table 1. Among the 7,602 women, the average age at oocyte retrieval was 32.28±4.85 years, the average body mass index (BMI) was 21.71±3.03 kg/m2 and the median duration of infertility was 3 years (interquartile range, 2–5); 5,409 of these women had regular menstruation (71.15%), 1,314 women had irregular periods (17.28%) and 879 were missing data (11.56%); 3,538 of these women had previous pregnancies (46.54%) resulting in 730 deliveries (9.60%), while 4,064 of these women never had a pregnancy before (53.46%). Indicators for IVF/ICSI treatments were tubal factor (49.75%), anovulation (3.59%), endometriosis (2.37%), male factor (11.81%), unexplained (1.71%), other (3.79%) and mixed factor (26.98%). The mean time of previous IVF failures was 0 (0–1). For the ovarian stimulation cycle, the median of total dose and duration of gonadotrophin was 1,800 IU (interquartile range, 1,500–2,025) and 9 days (interquartile range, 8–10), respectively. The median number of oocytes collected was 9 (interquartile range, 5–15), and median ovarian sensitivity index (OSI) was 1.65 (interquartile range, 1.20–2.08). For the method of fertilization, 5,249 of all included patients performed IVF (69.05%) and 2,353 of those performed ICSI (30.95%). This created a median number of 6 embryos (interquartile range, 4–10) and a median number of 4 frozen embryos (interquartile range, 2–6). Of those frozen embryos, the median number of cleavage stage and blastocyst stage embryos was 3 (interquartile range, 2–5) and 0 (interquartile range, 0–1), respectively.
Table 1
| Characteristics | Data |
|---|---|
| No. of IVF/ICSI cycles | 7,602 |
| No. of female | 7,602 |
| Patient characteristics | |
| Age at oocyte retrieval (year)★ | 32.28±4.85 |
| BMI (kg/m2)★ | 21.71±3.03 |
| Duration of infertility (years)▲ | 3 [2–5] |
| No previous pregnancy in couple, n (%) | 4,064 (53.46) |
| Previous pregnancy in couple, n (%) | 3,538 (46.54) |
| Previous delivery, n (%) | 730 (9.60) |
| Infertility etiology, n (%) | |
| Tubal | 3,782 (49.75) |
| Anovulatory | 273 (3.59) |
| Endometriosis | 180 (2.37) |
| Male factor | 898 (11.81) |
| Unexplained | 130 (1.71) |
| Other causes | 288 (3.79) |
| >1 type | 2,051 (26.98) |
| No. of previous IVF failures | 0 [0–1] |
| Year of oocytes retrieval, n (%) | |
| 2015 | 108 (1.42) |
| 2016 | 420 (5.52) |
| 2017 | 868 (11.42) |
| 2018 | 1,662 (21.86) |
| 2019 | 2,109 (27.74) |
| 2020 | 2,435 (32.03) |
| Treatment characteristics of ovarian stimulation cycle | |
| Total dose of gonadotrophin (IU)▲ | 1,800 (1,500–2,025) |
| Total duration of stimulation (days)▲ | 9 [8–10] |
| OSI▲ | 1.65 (1.20–2.08) |
| No. of oocytes collected▲ | 9 [5–15] |
| Method of fertilization, n (%) | |
| IVF | 5,249 (69.05) |
| ICSI | 2,353 (30.95) |
| No. of embryos created▲ | 6 [4–10] |
| No. of embryos frozen▲ | 4 [2–6] |
| Cleavage stage | 3 [2–5] |
| Blastocyst stage | 0 [0–1] |
★, values are presented as mean ± SD; ▲, values are presented as median (interquartile range). OSI = log (number of oocytes collected ×1,000/total dose of gonadotropin). OSI is a composite variable to measure ovarian response. IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; BMI, body mass index; OSI, ovarian sensitivity index; SD, standard deviation.
Characteristics of FET cycles
Cycle characteristics of FET were summarized in Table 2. The patients underwent a maximum of 4 FET cycles. Among the 9,964 FETs, endometrial preparation with induced cycle was used in more FET cycles (3,905 cycles, 39.19%). The remaining FETs prepared the endometrium with a natural cycle (2,766 cycles, 27.76%) or a hormonal substitution cycle (3,293 cycles, 33.05%). These endometrial preparations resulted in a median EMT of 10.80 (interquartile range, 9.40–12.40) mm. The heterogeneous, homogeneous and trilinear endometrial patterns were determined, respectively, as 40.90% (4,075/9,964), 51.12% (5,094/9,964) and 5.9% (588/9,964). In addition, 2.08% (207/9,964) of all cases lacked the data for endometrial pattern. The mean serum estradiol (E2) and P concentration on the day of transplantation were 174 (interquartile range, 105–258) pg/mL and 16.6 (interquartile range, 11.3–22.5) ng/mL, respectively. E2/P ratio on transplantation day was calculated from these data giving a median value of 10.86 (interquartile range, 5.73–20.27). The median value of storage duration, number of thawed and damaged thawed embryos, number of embryos transferred and number of top quality embryos transferred were 124 (interquartile range, 76–182) days, 2 (interquartile range, 2–2), 0 (interquartile range, 0–0), 2 (interquartile range, 2–2) and 0 (interquartile range, 0–1). In the majority of FET cycles, cleavage stage embryos were transferred. The median number of cleavage stage embryos transferred in all FET cycles was 2 (interquartile range, 2–2), while the median number of blastocyst stage embryos transferred was 0 (interquartile range, 0–1).
Table 2
| Characteristics | Data |
|---|---|
| No. of FET cycles | 9,964 |
| No. of frozen treatments | |
| 1 | 7,602 (68.94) |
| 2 | 1,931 (19.38) |
| 3 | 370 (3.71) |
| 4 | 61 (0.61) |
| Type of FET cycles, n (%) | |
| Natural cycle | 2,766 (27.76) |
| Induced cycle | 3,905 (39.19) |
| Hormonal substitution cycle | 3,293 (33.05) |
| EMT (mm)★ | 10.80 (9.40–12.40) |
| Endometrial pattern, n (%) | |
| Heterogeneous pattern | 4,075 (40.90) |
| Homogeneous pattern | 5,094 (51.12) |
| Trilaminar pattern | 588 (5.90) |
| Missing | 207 (2.08) |
| E2 level on transplantation day (pg/mL)▲ | 174 (105–258) |
| P level on transplantation day (ng/mL)★ | 16.6 (11.3–22.5) |
| E2/P on transplantation day▲ | 10.86 (5.73–20.27) |
| Storage duration (days)★ | 124 (76–182) |
| Embryos thawed (n)▲ | 2 (2–2) |
| Damaged thawed embryos (n)▲ | 0 (0–0) |
| Embryos transferred (n)▲ | 2 (2–2) |
| Cleavage stage (n)▲ | 2 (2–2) |
| Blastocyst stage (n)▲ | 0 (0–1) |
| Top quality embryos transferred (n)▲ | 0 (0–1) |
★, values are presented as mean ± SD; ▲, values are presented as median (interquartile range). FET, frozen-thawed embryo transfer; EMT, endometrial thickness; E2, estradiol; P, progesterone; SD, standard deviation.
Treatment outcomes
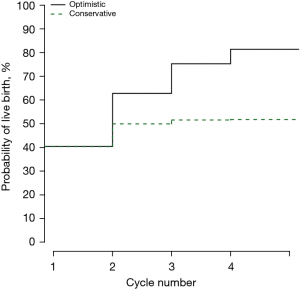
Of all FET cycles, the chance of having a child was 39.43% (3,929/9,964). The optimal and conservative estimates of CLBRs were 81.30% (95% CI: 80.41–82.17%) and 51.68% (95% CI: 50.55–52.81%), respectively. The LBR for the first FET cycle was 40.33% (95% CI: 39.23–41.44%), the subsequent FET cycles were correlated with lower LBRs [37.49% (95% CI: 35.33–39.70%), 33.51% (95% CI: 28.72–38.58%) and 24.59% (95% CI: 14.46–37.29%), for FET cycle 2 to 4, respectively (see Table 3)]. Of the remaining cycles, the differences of conservative CLBRs were small. The specific values were 49.86% (95% CI: 48.72–50.99%), 51.49% (95% CI: 50.36–52.62%) and 51.68% (95% CI: 50.55–52.81%), respectively in cycle 2–4. However, the optimal CLBRs were continued to increase from cycle 2 to 4 [62.70% (95% CI: 61.60–63.79%), 75.20% (95% CI: 74.22–76.17%) and 81.30% (95% CI: 80.41–82.17%), respectively (see Table 4)].
Table 3
| Characteristics | Data |
|---|---|
| FET 1, n (%) | |
| Positive β-hCG | 4,157/7,602 (54.68) |
| Preclinical losses to follow-up | 120/4,157 (2.89) |
| Biochemical pregnancies (preclinical losses) | 203/7,602 (2.67) |
| Ectopic pregnancy | 112/7,602 (1.47) |
| Clinical pregnancies | 3,722/7,602 (48.96) |
| Clinical losses to follow-up | 55/3,722 (1.48) |
| Abortion <12 weeks of gestation | 442/3,722 (11.88) |
| Abortion >12 weeks of gestation | 151/3,722 (4.06) |
| Live births | 3,066/7,602 (40.33) |
| Still births | 8/7,602 (0.11) |
| Deliveries | |
| Singleton | 2,211/3,074 (71.93) |
| Twin | 854/3,074 (27.78) |
| Triplet | 9/3,074 (0.29) |
| FET 2, n (%) | |
| Positive β-hCG | 985/1,931 (51.00) |
| Preclinical losses to follow-up | 21/985 (2.13) |
| Biochemical pregnancies (preclinical losses) | 61/1,931 (3.16) |
| Ectopic pregnancy | 32/1,931 (1.66) |
| Clinical pregnancies | 871/1,931 (45.11) |
| Clinical losses to follow-up | 13/871 (1.49) |
| Abortion <12 weeks of gestation | 101/871 (11.60) |
| Abortion >12 weeks of gestation | 31/871 (3.56) |
| Live births | 724/1,931 (37.49) |
| Still births | 2/1,931 (0.10) |
| Deliveries | |
| Singleton | 540/726 (74.38) |
| Twin | 186/726 (25.62) |
| Triplet | 0 |
| FET 3, n (%) | |
| Positive β-hCG | 178/370 (48.11) |
| Preclinical losses to follow-up | 5/178 (2.81) |
| Biochemical pregnancies (preclinical losses) | 13/370 (3.51) |
| Ectopic pregnancy | 8/370 (2.16) |
| Clinical pregnancies | 152/370 (41.08) |
| Clinical losses to follow-up | 2/152 (1.32) |
| Abortion <12 weeks of gestation | 23/152 (15.13) |
| Abortion >12 weeks of gestation | 3/152 (1.97) |
| Live births | 124/370 (33.51) |
| Still births | 0 |
| Deliveries | |
| Singleton | 94/124 (75.81) |
| Twin | 30/124 (24.19) |
| Triplet | 0 |
| FET 4, n (%) | |
| Positive β-hCG | 22/61 (36.07) |
| Preclinical losses to follow-up | 0 |
| Biochemical pregnancies (preclinical losses) | 1/61 (1.64) |
| Ectopic pregnancy | 0 |
| Clinical pregnancies | 21/61 (34.43) |
| Clinical losses to follow-up | 2/21 (9.52) |
| Abortion <12 weeks of gestation | 4/20 (20.00) |
| Abortion >12 weeks of gestation | 0 |
| Live births | 15/61 (24.59) |
| Still births | 0 |
| Deliveries | |
| Singleton | 12/15 (80.00) |
| Twin | 3/15 (20.00) |
| Triplet | 0 |
| Total FETs, n (%) | |
| Positive β-hCG | 5,342/9,964 (53.61) |
| Preclinical losses to follow-up | 146/5,342 (2.73) |
| Biochemical pregnancies (preclinical losses) | 278/9,964 (2.79) |
| Ectopic pregnancy | 152/9,964 (1.53) |
| Clinical pregnancies | 4,766/9,964 (47.83) |
| Clinical losses to follow-up | 72/4,766 (1.51) |
| Abortion <12 weeks of gestation | 570/4,766 (11.96) |
| Abortion >12 weeks of gestation | 185/4,766 (3.88) |
| Live births | 3,929/9,964 (39.43) |
| Still births | 10 |
| Deliveries | |
| Singleton | 2,857/3,939 (72.53) |
| Twin | 1,073/3,939 (27.24) |
| Triplet | 9/3,939 (0.23) |
All pregnancies are counted, showing the total reproductive outcome. Twins are counted as one live birth. FET, frozen-thawed embryo transfer; hCG, human chorionic gonadotropin.
Table 4
| Cycle No. | No. of cycles | No. of live births | LBR within each cycle, % (95% CI) | CLBRs across all FET cycles, % (95% CI) | |
|---|---|---|---|---|---|
| Optimal estimatea | Conservative estimateb | ||||
| 1 | 7,602 | 3,066 | 40.33 (39.23, 41.44) | 40.33 (39.23, 41.44) | 40.33 (39.23, 41.44) |
| 2 | 1,931 | 724 | 37.49 (35.33, 39.70) | 62.70 (61.60, 63.79) | 49.86 (48.72, 50.99) |
| 3 | 370 | 124 | 33.51 (28.72, 38.58) | 75.20 (74.22, 76.17) | 51.49 (50.36, 52.62) |
| 4 | 61 | 15 | 24.59 (14.46, 37.29) | 81.30 (80.41, 82.17) | 51.68 (50.55, 52.81) |
a, it was based on the assumption that women who discontinued treatment would have had the same chance of a pregnancy resulting in a live birth as those who remained in treatment; b, it was based on the assumption that none of the women who discontinued treatment would have had a live birth. LBR, live-birth rate; FET, frozen-thawed embryo transfer; CLBRs, cumulative live birth rates; CI, confidence interval.
LBRs and CLBRs varied when female age at oocyte retrieval, number of oocytes collected and endometrial thickness (EMT) on the day of embryos transferred were different (Table S1).
Predictors of the CLBRs
Among the categories collected before and during treatment, all characteristics had statistically significant univariate associations with LBRs, except for the method of fertilization, P level and E2/P ratio on transplantation day, embryos transferred and number of top-quality embryos (Table S2).
Table 5 presents the best prediction model which contained eight predictors using the multivariate analysis. The factors that predicted the cumulative rates of having a live birth over a complete “freeze-all” policy were the woman’s age at oocyte retrieval, EMT, number of oocytes, previous IVF failures, number of embryos frozen, duration of infertility and number of blastocysts transferred. The odds of a live birth decreased with every increasing number of FET cycle which derived from one episode of ovarian stimulation.
Table 5
| Parameter | Value | OR (95% CI) | P value | Adequacy |
|---|---|---|---|---|
| FET cycle number | 1 (reference) | 1 | NA | |
| 2 | 0.891 (0.741, 1.071) | 0.218 | ||
| 3 | 0.609 (0.402, 0.923) | 0.019 | ||
| 4 | 0.235 (0.061, 0.913) | 0.036 | ||
| EMT | ≤8 mm (reference) | 1 | 0.938 | |
| >8 mm, ≤12 mm | 1.423 (1.006, 2.013) | 0.046 | ||
| >12 mm | 1.777 (1.261, 2.505) | <0.001 | ||
| Age at oocyte retrieval | <31 years (reference) | 1 | 0.082 | |
| 31–35 years | 0.779 (0.672, 0.903) | <0.001 | ||
| 36–40 years | 0.516 (0.411, 0.648) | <0.001 | ||
| >40 years | 0.125 (0.074, 0.210) | <0.001 | ||
| No. of oocytes collected | 0–5 (reference) | 1 | 0.027 | |
| 6–10 | 0.900 (0.734, 1.105) | 0.314 | ||
| 11–15 | 0.968 (0.768, 1.220) | 0.780 | ||
| 16–20 | 0.742 (0.564, 0.975) | 0.032 | ||
| >20 | 0.895 (0.651, 1.230) | 0.493 | ||
| No. of previous IVF failures | 0.921 (0.876, 0.967) | <0.001 | 0.025 | |
| No. of embryos frozen | Blastocyst stage | 1.078 (1.020, 1.140) | 0.008 | 0.022 |
| Cleavage stage | 1.062 (1.020, 1.104) | 0.003 | 0.017 | |
| Duration of infertility | 0.968 (0.944, 0.994) | 0.015 | 0.014 | |
| No. of blastocyst transferred | 1.446 (1.229, 1.701) | <0.001 | 0.006 |
ORs, odds ratios; CIs, confidence intervals; FET, frozen-thawed embryo transfer; EMT, endometrial thickness; IVF, in vitro fertilization.
Figure 2 shows CLBRs stratified according to categorical variables of positive significance in the multivariate model. Figure 2A,2B show the respective conservative and optimistic CLBRs stratified according to EMT. Figure 2C,2D show the conservative and optimistic CLBRs stratified according to female age at oocyte retrieval, respectively. Figure 2E,2F show the conservative and optimistic CLBRs stratified according to the number of oocytes collected, respectively.
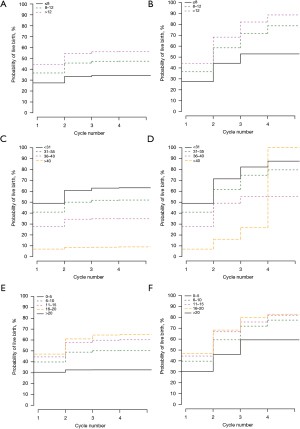
Figure 3 shows the adjusted (multivariate model) relation between the predicted likelihood of live birth with the “freeze-all” policy and the number of previous IVF failures, duration of infertility, number of cleavage- and blastocyst-stage embryos frozen, and number of blastocysts transferred by using restricted cubic splines analysis. The probability of a live birth decreased linearly with increasing number of previous IVF failures and duration of infertility. Increasing numbers of blastocysts transferred, cleavage- and blastocyst-stage embryos frozen improved the chance of live birth.
Assessing the ability of the prediction model
The c-statistic for the model in the training set was 0.67 and was similar to the validation set (0.68). The calibration slope as assessed by the Hosmer-Lemeshow test, showed a P value of >0.05 in both sets (0.94 and 0.16, respectively) indicating no overfitting of predictor effects.
Discussion
As there are increasing concerns about the adverse effects of controlled ovarian stimulation (COS) (30) combined with fresh embryo transfer (ET) therapies, the “freeze-all” strategy (defined as the entire cohort of fresh embryos being cryopreserved in an IVF or ICSI cycle followed by the frozen-thawed embryos transfer in later cycles with improved embryo cryopreservation techniques), has gradually attracted the attention of many reproductive centers around the world. Although the freeze-all policy seems to be an ideal alternative to fresh ET as it has some potential advantages (3,31), it remains unclear what the long-term reproductive consequences will be with regards to LBR. Previous studies on the chance of a live birth after IVF or ICSI (16,32,33), have either made predictions for a complete package of IVF cycle including all fresh and FETs, or made predictions for live birth after IVF/ICSI treatment but with single-embryo transfer (SET) after 2 days of embryo culture (33). A recent study by Chang et al. (34) reported the comparison of CLBRs between the “freeze-all” population (study group) and “fresh ET” population (control group) during the period from January 2012 to June 2014. The authors found that the CLBRs in two groups were significantly different (64.3% in study group vs. 45.8% in control group). Furthermore, the CLBRs in the study group were significantly higher (58.3% vs. 40.9%) when the number of eggs is between 4 and 15. Unsurprisingly, they came to the conclusion that the “freeze-all policy” improved the assisted reproductive technology (ART) outcome for normal responders. Our clinic found that the chance of having a child after the first complete IVF cycle was 50.74% with the freeze-all strategy (33). However, Chang et al. (34) neither constructed a predictive model to evaluate the risk factors which may affect the LBR with the “freeze-all” policy or explained the optimistic and pessimistic CLBRs, between which the realistic cumulative rates may exist.
The goal of this study was to elucidate the predictors of LBR with “freeze-all” policy over an entire IVF/ICSI treatment course and calculate meaningful CLBRs to answer a couple’s most concerned question—what is the chance that the “freeze-all” policy will result in a baby? As demonstrated in previous studies where the CLBR does not vary substantively with the indication for ART treatment (35,36), we did not exclude women on the basis of age, BMI, ovarian-reserve function, or other prognostic factors except uterine abnormality. Thus, the inclusion of all subjects who presented for their first ovarian stimulation treatment and underwent “freeze-all” policy in our center increases the generalizability of our model outcomes. In total, 9,964 IVF/ICSI-FET cycles were performed in 7,602 couples and resulted in 3,929 live births. The CLBR in the study population varied from 51.68% (95% CI: 50.55–52.81%) to 81.30% (95% CI: 80.41–82.17%) after four consecutive cycles of FET, and those values corresponded to the optimistic and conservative estimate, respectively. As expected, among the couples embarking on IVF or ICSI we found that the EMT on the day of embryo transferred was far the best predictor of live birth when the treatment procedure was carried out to the frozen-thawed cycles. Additionally, female age at oocytes retrieval, number of eggs collected, previous IVF failures, duration of infertility and the cryopreservation of embryos were the next best predictors.
A number of current reviews and meta-analyses have summarized the available knowledge concerning the association of EMT with the chance of achieving a pregnancy or live birth after IVF, but with conflicting results. Most of the previous studies found an association between EMT and pregnancy or live birth (37-40). Especially for some retrospective studies, it was found that EMT significantly affected the LBRs either in fresh autologous IVF cycle or FET cycle (39,40). In addition, one study reporting on EMT in natural FET cycles showed that pregnancy rates were significantly lower in patients with suboptimal endometrial development, suggesting an independent predictive role of EMT (41). However, a significant correlation between EMT and the chance to conceive or have a live birth has failed to be established by other authors (42-44). They concluded that the EMT is a poor predictor of IVF success and has a limited capacity for the occurrence of pregnancy while acknowledging that below a cut-off of 7 millimeters (mm), a lower chance of pregnancy can be observed in univariate analyses. In our study, EMT was assigned to the following categories: < or =8, (8,12) and >12 mm. These intervals were chosen according to previous studies (45,46) to facilitate the application of the results towards everyday clinical practice. We found that EMT < or =8 mm was associated with an obvious decrease in outcomes and it was a significant predictor for LBRs, results that are more in line with the conclusion of at least one previous study with a higher incidence of pregnancy rates as EMT increased from the category of <8 to 8–12 and >12 mm as well (46). In addition to EMT, we also analyzed the effects of different endometrial preparation and endometrial pattern on LBRs, but no significant correlation was found. This outcome is consistent with the results of other studies (45). On the basis of these results, one could conclude that pregnancy prospects may improve with a thicker EMT, yet it is unclear why thin EMT is associated with poorer IVF outcomes.
There is a natural trend of age-related decline in fecundability, not only in the population surveyed in previous studies (47,48), but also in our cohort. Not surprisingly, we found that maternal age which is the most established predictor included in every prediction model for the success of IVF/ICSI (49,50) was negatively correlated with live birth. Women who were 40 years of age or younger and treated with up to 4 cycles of FET could achieve CLBRs that were similar to or even higher than published cumulative pregnancy proportion achieved naturally at 6 menstrual cycles (49). However, there’s hardly any difference in the LBR both in our subjects and the above population once women are over 40 years of age. This suggests that IVF/ICSI-FET overcomes infertility in younger women but cannot completely reverse the age-dependent decrease in fertility for women older than 40 years. Thus, it is advisable to consider female age in individualized counselling before initiating an IVF journey.
A longstanding concern involves the importance of the total number of oocytes retrieved in models that predict pregnancy or live birth (51,52). Similar to previous studies (53), we found that LBR increases with increasing egg number in IVF cycles. However, LBR was significantly improved only when 16–20 oocytes were retrieved. Moreover, when >15 oocytes were retrieved, LBR almost plateaus and this is in line with the results of other studies (54,55). Due to the “freeze-all” policy, there was no late-onset OHSS occurrence. And there were only seven early-onset moderate OHSS and no severe OHSS in this dataset. Given the apparent relationship between high retrieved oocyte number and plateaued increase in LBR, it seems reasonable that optimal number of oocytes should be the pursuit of less aggressive stimulation protocols.
As well-known predictors for live birth, the number of previous IVF failures and duration of infertility were examined in the present study. Our findings are consistent with previous work showing that women who have less experience of IVF failures (56) and shorter duration of infertility have a better chance of subsequent live birth (57). It is speculated that those couples with fewer unsuccessful IVF journeys and a shorter duration of infertility are likely affected by fewer or less severe fertility factors and therefore may be expected to have superior outcomes after IVF. There is no doubt that the more eggs are obtained, the greater the probability of getting the available embryos and the higher the CLBRs is. And this has been confirmed in the present study that LBR increases when more embryo cryopreservation is available. To a certain extent, our finding is similar to other research (58), in that the number of blastocysts transferred correlated positively with the odds of live birth. Despite the number of blastocyst transfers having lower impact in the variation of the whole regression, it remained significant. This could be due to the much larger sample size in our data set.
It is worth noting that no obvious relationship between embryo quality and LBR was found and this is not consistent with other studies (33,59). Part of this may be related to the embryo selection strategy used in our center. We routinely freeze top-quality embryos, and the remaining embryos are cultured for blastocysts. Therefore, patients who are able to enter the FET cycle almost always have good quality embryos for transplantation; only those who have great difficulties in getting embryos will freeze and transfer the inferior embryos. This weakens the role of embryo quality in predicting live birth to a certain extent. On the other hand, our study is not based on elective SET which may contribute a more accurate inference to the relationship between embryo quality and live birth. Other parameters such as woman’s age at FET, duration and total dosage of Gn, OSI and cleavage number were all excluded from analysis due to their collinearity with observed variables—e.g., the woman’s age at oocyte retrieval and number of eggs collected that were always included and could not be removed because these are known predictors of pregnancy outcomes after IVF.
An advantage of our study is that the number of patients is sufficient for model construction and that the model is developed from a large number of variables recorded before and during the IVF treatment within a single clinic. Previous prediction models for LBR after IVF treatment have presented C-index of 0.68–0.73 and P value in the Hosmer-Lemeshow test of 0.88–0.998 (16,33). Referring to these data, the discriminative capacity of the present model was modest, while the test for calibration was excellent in the training set but not in the validation set. This indicated that there is a good parallelism between the predicted chance of success and the observed success rate in subjects. Although the conventional relationship between EMT and the chance of achieving a live birth after IVF is not consistent across studies, the role of EMT as an expected variable has been strengthened by our predictive model when the “freeze-all” policy is carried out. The results from our model are relevant both for individual couples and their clinicians. Couples who would ideally like to know their overall chance of having a baby over a complete IVF procedure when they are fit for the “freeze-all” policy might be separately counselled by using this model. As an example, the probability of a live birth in the first FET cycle for a woman older than 40 years is 6.83%, after two cycles of FET the conservative and optimal CLBRs increase to 8.59% and 26.4%, respectively. Irrespective of age, the probability of a live birth in the first FET cycle is 27.4% when one’s EMT reached 8 mm, after a complete package of FET cycles the conservative and optimal CLBRs increase to 34.25% and 52.83%, respectively. Therefore, clinicians would use these predictions to support their clinical knowledge when communication with couples before the “freeze-all” policy is started.
Some limitations of this study need to be addressed. Although we successfully established a prediction model, all variables introduced in both univariate and multivariate analyses were retrospectively collected in study. Therefore, there probably is an inevitable risk of bias. It must also be mentioned that data collection in a single clinic while avoiding the heterogeneity of a cohort of managed patients on the one hand, can be a double-edged sword for the applicability to other IVF centers. Ideally, a prediction model for IVF should include potentially important predictors, such as: smoking, alcohol intake and complications like occurrence of COS, which were not available in our dataset and we were unable to adjust for. As a consequence, it may be inappropriate to make conclusions about the influence of potential confounders for the LBR. Moreover, stratification (such as female age and EMT individually stratified instead of multifactor stratification) in analyses was only performed for univariate analyses as the available data wasn’t sufficient. It is worth looking forward to reveal the possible effects of the predictors on LBR under the interaction of predictors. These limitations of the present study must guide future studies.
Conclusions
In conclusion, this study has developed a novel model that can predict the cumulative chances of a live birth for an individual couple over an entire course of IVF cycle undergoing the “freeze-all” strategy. We confirmed female age at oocytes retrieval, previous IVF failures, duration of infertility, number of eggs collected, number of cleavage- and blastocyst-stage embryos frozen, EMT and number of blastocyst transferred as independent predictors. Not surprisingly, the chance of live birth increases with optimal endometrial development, increasing eggs, increasing cryopreserved embryos and increasing number of blastocyst transferred, whereas decreases with increasing age, increasing number of previous IVF failures and increasing duration of infertility. It is suggested that the present prediction model will help to facilitate couples’ counselling before and during IVF with the “freeze-all” policy.
Acknowledgments
We especially thank Ian Max Andolina, PhD for helping us improve the English expression and gratefully acknowledge all staff of the Department of Assisted Reproduction in Shanghai First Maternity and Infant Hospital for their support and cooperation.
Funding: The present study was supported by the National Nature Science Foundation of China (grant No. 81501319), the Special Funds for Clinical Medical Research of Chinese Medical Association (No. 18010030732) and the Foundation of Health and Family Planning Commission of Shanghai (grant No. 201540237).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-589/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-589/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-589/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Shanghai First Maternity and Infant Hospital of Tongji University School of Medicine (No. KS21282) and individual informed consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zeilmaker GH, Alberda AT, van Gent I, et al. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril 1984;42:293-6. [Crossref] [PubMed]
- Herrero L, Martínez M, Garcia-Velasco JA. Current status of human oocyte and embryo cryopreservation. Curr Opin Obstet Gynecol 2011;23:245-50. [Crossref] [PubMed]
- Roque M, Valle M, Guimarães F, et al. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril 2015;103:1190-3. [Crossref] [PubMed]
- Roque M. Freeze-all policy: is it time for that? J Assist Reprod Genet 2015;32:171-6. [Crossref] [PubMed]
- Abuzeid MI, Bolonduro O, La Chance J, et al. Cumulative live birth rate and assisted reproduction: impact of female age and transfer day. Facts Views Vis Obgyn 2014;6:145-9. [PubMed]
- Wong KM, van Wely M, Mol F, et al. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2017;3:CD011184. [Crossref] [PubMed]
- Imudia AN, Awonuga AO, Kaimal AJ, et al. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertil Steril 2013;99:168-73. [Crossref] [PubMed]
- Maheshwari A, Bhattacharya S. Elective frozen replacement cycles for all: ready for prime time? Hum Reprod 2013;28:6-9. [Crossref] [PubMed]
- Chen ZJ, Shi Y, Sun Y, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med 2016;375:523-33. [Crossref] [PubMed]
- Shi Y, Sun Y, Hao C, et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. N Engl J Med 2018;378:126-36. [Crossref] [PubMed]
- Vuong LN, Dang VQ, Ho TM, et al. IVF Transfer of Fresh or Frozen Embryos in Women without Polycystic Ovaries. N Engl J Med 2018;378:137-47. [Crossref] [PubMed]
- McLernon DJ, Maheshwari A, Lee AJ, et al. Cumulative live birth rates after one or more complete cycles of IVF: a population-based study of linked cycle data from 178,898 women. Hum Reprod 2016;31:572-81. [Crossref] [PubMed]
- Moragianni VA, Penzias AS. Cumulative live-birth rates after assisted reproductive technology. Curr Opin Obstet Gynecol 2010;22:189-92. [Crossref] [PubMed]
- Kupka MS, Ferraretti AP, de Mouzon J, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE†. Hum Reprod 2014;29:2099-113. [Crossref] [PubMed]
- Smith ADAC, Tilling K, Nelson SM, et al. Live-Birth Rate Associated With Repeat In Vitro Fertilization Treatment Cycles. JAMA 2015;314:2654-62. [Crossref] [PubMed]
- McLernon DJ, Steyerberg EW, Te Velde ER, et al. Predicting the chances of a live birth after one or more complete cycles of in vitro fertilisation: population based study of linked cycle data from 113 873 women. BMJ 2016;355:i5735. [Crossref] [PubMed]
- Shapiro BS, Daneshmand ST, Garner FC, et al. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril 2011;96:516-8. [Crossref] [PubMed]
- Shapiro BS, Daneshmand ST, Garner FC, et al. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011;96:344-8. [Crossref] [PubMed]
- Ozgur K, Bulut H, Berkkanoglu M, et al. Prediction of live birth and cumulative live birth rates in freeze-all-IVF treatment of a general population. J Assist Reprod Genet 2019;36:685-96. [Crossref] [PubMed]
- Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril 2014;101:105-11. [Crossref] [PubMed]
- Kuang Y, Chen Q, Hong Q, et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online 2014;29:684-91. [Crossref] [PubMed]
- Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril 2015;104:62-70.e3. [Crossref] [PubMed]
- Cummins JM, Breen TM, Harrison KL, et al. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf 1986;3:284-95. [Crossref] [PubMed]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod 2009;24:2683-7. [Crossref] [PubMed]
- Maul A. A discrete time logistic regression model for analyzing censored survival data. Environmetrics 1994;5:145-57. [Crossref]
- Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem 2008;54:17-23. [Crossref] [PubMed]
- Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128-38. [Crossref] [PubMed]
- Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011;30:1105-17. [Crossref] [PubMed]
- Harrell FE Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Cham: Springer, 2015.
- Roque M, Lattes K, Serra S, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril 2013;99:156-62. [Crossref] [PubMed]
- Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril 2014;102:19-26. [Crossref] [PubMed]
- Dhillon RK, McLernon DJ, Smith PP, et al. Predicting the chance of live birth for women undergoing IVF: a novel pretreatment counselling tool. Hum Reprod 2016;31:84-92. [Crossref] [PubMed]
- Vaegter KK, Lakic TG, Olovsson M, et al. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil Steril 2017;107:641-8.e2. [Crossref] [PubMed]
- Chang JC, Chen MJ, Guu HF, et al. Does the "freeze-all" policy allow for a better outcome in assisted reproductive techniques than the use of fresh embryo transfers? - A retrospective study on cumulative live birth rates. Taiwan J Obstet Gynecol 2017;56:775-80. [Crossref] [PubMed]
- Stolwijk AM, Wetzels AM, Braat DD. Cumulative probability of achieving an ongoing pregnancy after in-vitro fertilization and intracytoplasmic sperm injection according to a woman's age, subfertility diagnosis and primary or secondary subfertility. Hum Reprod 2000;15:203-9. [Crossref] [PubMed]
- Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med 2009;360:236-43. [Crossref] [PubMed]
- Kovacs P, Matyas S, Boda K, et al. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod 2003;18:2337-41. [Crossref] [PubMed]
- Traub ML, Van Arsdale A, Pal L, et al. Endometrial thickness, Caucasian ethnicity, and age predict clinical pregnancy following fresh blastocyst embryo transfer: a retrospective cohort. Reprod Biol Endocrinol 2009;7:33. [Crossref] [PubMed]
- Bu Z, Wang K, Dai W, et al. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol Endocrinol 2016;32:524-8. [Crossref] [PubMed]
- Holden EC, Dodge LE, Sneeringer R, et al. Thicker endometrial linings are associated with better IVF outcomes: a cohort of 6331 women. Hum Fertil (Camb) 2018;21:288-93. [Crossref] [PubMed]
- Basir GS. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet Gynecol 2002;19:484-9. [Crossref] [PubMed]
- Griesinger G, Trevisan S, Cometti B. Endometrial thickness on the day of embryo transfer is a poor predictor of IVF treatment outcome. Hum Reprod Open 2018;2018:hox031. [Crossref] [PubMed]
- Kasius A, Smit JG, Torrance HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 2014;20:530-41. [Crossref] [PubMed]
- Barker MA, Boehnlein LM, Kovacs P, et al. Follicular and luteal phase endometrial thickness and echogenic pattern and pregnancy outcome in oocyte donation cycles. J Assist Reprod Genet 2009;26:243-9. [Crossref] [PubMed]
- Rashidi BH, Sadeghi M, Jafarabadi M, et al. Relationships between pregnancy rates following in vitro fertilization or intracytoplasmic sperm injection and endometrial thickness and pattern. Eur J Obstet Gynecol Reprod Biol 2005;120:179-84. [Crossref] [PubMed]
- Aboulghar MM, Mansour RT, Al-Inany HG, et al. Three dimensional endometrial volume versus endometrial thickness measurement in prediction of IVF/ICSI outcome. Middle East Fertility Society Journal 2005;10:63-7.
- Wesselink AK, Rothman KJ, Hatch EE, et al. Age and fecundability in a North American preconception cohort study. Am J Obstet Gynecol 2017;217:667.e1-8. [Crossref] [PubMed]
- McPherson NO, Zander-Fox D, Vincent AD, et al. Combined advanced parental age has an additive negative effect on live birth rates-data from 4057 first IVF/ICSI cycles. J Assist Reprod Genet 2018;35:279-87. [Crossref] [PubMed]
- van Loendersloot LL, van Wely M, Repping S, et al. Individualized decision-making in IVF: calculating the chances of pregnancy. Hum Reprod 2013;28:2972-80. [Crossref] [PubMed]
- Choi B, Bosch E, Lannon BM, et al. Personalized prediction of first-cycle in vitro fertilization success. Fertil Steril 2013;99:1905-11. [Crossref] [PubMed]
- Sunkara SK, Rittenberg V, Raine-Fenning N, et al. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 2011;26:1768-74. [Crossref] [PubMed]
- Ottosen LD, Kesmodel U, Hindkjaer J, et al. Pregnancy prediction models and eSET criteria for IVF patients--do we need more information? J Assist Reprod Genet 2007;24:29-36. [Crossref] [PubMed]
- Fatemi HM, Doody K, Griesinger G, et al. High ovarian response does not jeopardize ongoing pregnancy rates and increases cumulative pregnancy rates in a GnRH-antagonist protocol. Hum Reprod 2013;28:442-52. [Crossref] [PubMed]
- Steward RG, Lan L, Shah AA, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril 2014;101:967-73. [Crossref] [PubMed]
- Baker VL, Brown MB, Luke B, et al. Association of number of retrieved oocytes with live birth rate and birth weight: an analysis of 231,815 cycles of in vitro fertilization. Fertil Steril 2015;103:931-8.e2. [Crossref] [PubMed]
- Tigges J, Godehardt E, Soepenberg T, et al. Determinants of cumulative ART live-birth rates in a single-center study: age, fertilization modality, and first-cycle outcome. Arch Gynecol Obstet 2016;294:1081-9. [Crossref] [PubMed]
- Pettersson G, Andersen AN, Broberg P, et al. Pre-stimulation parameters predicting live birth after IVF in the long GnRH agonist protocol. Reprod Biomed Online 2010;20:572-81. [Crossref] [PubMed]
- Hill MJ, Richter KS, Heitmann RJ, et al. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil Steril 2013;99:1631-6. [Crossref] [PubMed]
- Niinimäki M, Veleva Z, Martikainen H. Embryo quality is the main factor affecting cumulative live birth rate after elective single embryo transfer in fresh stimulation cycles. Eur J Obstet Gynecol Reprod Biol 2015;194:131-5. [Crossref] [PubMed]


