
The relationship between TSH levels and clinical pregnancy outcomes for patients who undergo in vitro fertilization/intracytoplasmic sperm injection: a retrospective study
Introduction
Thyroid-stimulating hormone (TSH) has been proposed as playing a role in reproductive difficulties over several decades (1), including ovulatory dysfunction, infertility, miscarriage, and adverse maternal complications. Several researches have linked the thyroid function to ovarian function and the physiology of reproduction. Thyroid hormone may influence the folliculogenesis, estrogen and androgen metabolism, the menstrual cycle (2,3), and endometrial receptivity (4).
TSH levels prior to pregnancy are between 2.5 and 4 mIU/L, and management options include either monitoring levels and treatment when TSH >4 mIU/L, or treating with levothyroxine to maintain TSH less than 2.5 mIU/L according to Grade C evidence based on international guidelines from the Practice Committee of the American Society for Reproductive Medicine (5). In an iodine sufficient area, an upper limit of a normal TSH threshold of 4.12 mIU/L should be considered. According to the Chinese Society for Reproductive Medicine consensus for subclinical hypothyroidism in the infertile female population, a TSH threshold of 4–4.5 mIU/L for infertile women and women attempting pregnancy should be considered (6).
Therefore, the suggested TSH threshold before women undergo in vitro fertilization (IVF) and embryo transfer should be 4 mIU/L. Infertile patients with untreated TSH >2.5 mIU/L before IVF/intracytoplasmic sperm injection (ICSI) have been examined in retrospective studies, and reported better pregnancy outcomes when TSH is <2.5 mIU/L compared with women with TSH <2.5 mIU/L (7,8). Both the Endocrine Society and the American Thyroid Association guidelines recommend TSH ranges from 0.1–2.5 mIU/L in the first trimester of pregnancy; 0.2–3.0 mIU/L in the second trimester; and 0.3–3.5 mIU/L in the third trimester (9,10). Some studies suggest the upper reference range of TSH may be approximately 2.5–3.1 mIU/L based on normative data of healthy pregnant women (11-13). Therefore, what the TSH threshold should be before women undergo IVF and embryo transfer remains controversial. We present the following article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-79/rc).
Methods
Study design
Patients undergoing fresh IVF/ICSI embryo transfer cycles for the first time who presented between January 1, 2015 and December 31, 2017 at the Chongqing Institute of Reproductive and Genetic, Chongqing Health Center for Women and Children were recruited into this retrospective cohort study. The center is the largest reproductive medicine center in southwestern China.
Study participants
All TSH and hormone levels were measured before patients underwent IVF/ICSI within 1 year. Patients with advanced reproductive age (≥40 years), severe underweight or obese status [body mass index (BMI) ≤18 or ≥28 kg/m2], the man with severe oligoasthenospermia, women with poor ovarian reserve, and presence of endocrine disorders (diabetes mellitus, hyperprolactinemia, congenital adrenal hyperplasia, or Cushing syndrome), uterine anomaly confirmed by either hysterosalpingography or hysteroscopy, sactosalpinx diagnosed by gynecological ultrasound, hystersalpingography or pelvic surgery, untreated hyperthyroidism (TSH <0.35 mIU/L), hypothyroidism (TSH >4.94 mIU/L) or abnormal serum FT3 and FT4 level, preimplantation genetic diagnosis, and chromosomal abnormality or polymorphism were excluded.
IVF/ICSI procedure
Patients underwent controlled ovarian stimulation accomplished with an individualized GnRH-agonist/GnRH-antagonist protocol. All patients underwent first fresh embryo transfers which were performed 3 days after oocyte retrieval.
Laboratory analysis
Serum TSH levels was measured by Chemiluminescent Microparticle ImmunoAssay, CMIA (Abbott Architect TSH, Abbott Architect i2000SR, South Kraemer Boulevard, CA). A normal range was allocated as 0.35–4.94 mIU/L, and the intra- and inter-assay coefficient of variation (CV) was ≤10%.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 statistical software (Chicago, IL, USA). Data are presented as mean ± SD. An independent-sample t-test was used for continuous variables where appropriate, and Chi-square or Fisher exact tests were used for categorical l variables. Receiver operating characteristic (ROC) curves were generated by plotting the sensitivity and 1-specificity of TSH values for all variables, while logical regression was used to reveal the predictors of clinical pregnancy outcome. All analyses of significance were two-sided and tested at the 5% level, and P<0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Chongqing Health Center for Women and Children Ethics Committee (No. 2022-RGI-02). Individual consent for this retrospective analysis was waived.
Results
We recruited 6,088 first time fresh transfer cycles, and the overall clinical pregnancy rate, live birth rate, and miscarriage rate were 58.65%, 51.15% and 12.95%, respectively. The area under the ROC curves for live birth was 0.496 (95% CI: 0.481–0.510), for clinical pregnancy 0.503 (95% CI: 0.488–0.517), and for miscarriage 0.523 (95% CI: 0.496–0.552) (Figure 1).
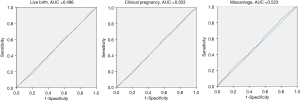
All three ROC curves approached the line of no discrimination, indicating there was no value of TSH within the normal range that could predict clinical pregnancy, live birth, or miscarriage in patients who underwent IVF/ICSI. As a cutoff value of TSH was not identified for clinical pregnancy outcomes in the ROC analysis, we divided patients into the following groups according to the serum TSH levels: 0.35 to ≤1, 1 to ≤1.5, 1.5 to ≤2, 2 to ≤2.5, 2.5 to ≤3, 3 to ≤3.5, 3.5 to ≤4, 4 to ≤4.5, and 4.5 to ≤4.94 mIU/L (Table 1). The live birth rate, clinical pregnancy rate and miscarriage rate were then evaluated among the groups, and the results showed there was a general downward trend in live birth rate and clinical pregnancy rate and an upward trend in the miscarriage rate at the cutoff value of TSH >3 mIU/L (Figure 2). However, as guidelines dictate a TSH level >4 mIU/L during pregnancy is associated with miscarriage, we analyzed the clinical pregnancy outcomes and general characteristics at the upper range of TSH at 3, 3.5, and 4 mIU/L (Tables 2-4).
Table 1
| TSH levels (mIU/L) | LBR (%) | CPR (%) | MR (%) |
|---|---|---|---|
| 0.35 to <1 | 325/646 (50.31) | 358/646 (55.42) | 33/358 (9.22) |
| 1 to <1.5 | 701/1,349 (51.96) | 802/1,349 (59.45) | 101/802 (12.59) |
| 1.5 to <2 | 720/1,382 (52.10) | 834/1,382 (60.35) | 114/834 (13.67) |
| 2 to <2.5 | 524/1,010 (51.88) | 596/1,010 (59.01) | 72/596 (12.08) |
| 2.5 to <3 | 367/705 (52.06) | 425/705 (60.28) | 58/425 (13.65) |
| 3 to <3.5 | 217/460 (47.17) | 253/460 (55.00) | 36/253 (14.23) |
| 3.5 to <4 | 125/261 (47.89) | 146/261 (55.94) | 21/146 (14.38) |
| 4 to <4.5 | 83/168 (49.40) | 98/168 (58.33) | 15/98 (15.31) |
| 4.5 to ≤4.94 | 52/107 (48.60) | 64/107 (59.81) | 12/64 (18.75) |
TSH, thyroid-stimulating hormone; LBR, live birth rate; CPR, clinical pregnancy rate; MR, miscarriage rate.
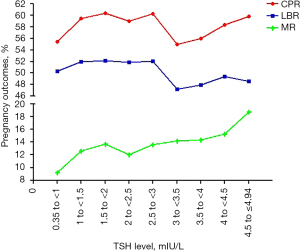
Table 2
| Characteristic | TSH ≤3 mIU/L | TSH >3 mIU/L | P value |
|---|---|---|---|
| No. of cycles | 5,092 | 996 | |
| Age (years) | 31.17±4.08 | 31.43±4.21 | 0.068 |
| Duration of infertility (years) | 5.78±3.90 | 5.95±3.70 | 0.205 |
| BMI (kg/m2) | 21.88±2.29 | 22.03±2.26 | 0.053 |
| Basal FSH (IU/L) | 5.77±1.91 | 5.62±1.76 | 0.653 |
| Basal E2 (pg/mL) | 41.39±130.43 | 45.39±202.03 | 0.495 |
| AMH (ng/mL) | 3.59±2.86 | 3.40±2.50 | 0.111 |
| TSH (mIU/L) | 1.73±0.62 | 3.70±0.53 | 0.000 |
| FT3 (pmol/L) | 4.39±0.46 | 4.41±0.47 | 0.659 |
| FT4 (pmol/L) | 13.51±1.50 | 13.32±1.52 | 0.594 |
| Duration of Gn (days) | 11.26±1.42 | 11.31±1.47 | 0.280 |
| Total Gn dose (IU) | 2,525.12±800.38 | 2,540.75±829.40 | 0.575 |
| No. of oocytes retrieved | 10.01±4.29 | 10.06±4.35 | 0.774 |
| No. of usable embryos (n) | 3.59±2.56 | 3.99±2.59 | 0.631 |
| Endometrial thickness on day of ET (mm) | 10.09±1.62 | 10.09±1.62 | 0.973 |
| Clinical pregnancy rate (%) | 3,015/5,092 (59.21%) | 561/996 (56.32%) | 0.091 |
| Miscarriage rate (%) | 378/3,015 (12.54%) | 84/561 (14.97%) | 0.114 |
| Live birth rate (%) | 2,637/5,092 (51.79%) | 477/996 (47.89%) | 0.024 |
TSH, thyroid-stimulating hormone; BMI, body mass index; FSH, follicle-stimulating hormone; E2, estradiol; AMH, anti-Mullerian hormone; ET, embryo transfer.
Table 3
| Characteristic | TSH ≤3.5 mIU/L | TSH >3.5 mIU/L | P value |
|---|---|---|---|
| No. of cycles | 5,552 | 536 | |
| Age (years) | 31.19±4.10 | 31.33±4.12 | 0.459 |
| Duration of infertility (years) | 5.79±3.90 | 5.94±3.57 | 0.369 |
| BMI (kg/m2) | 21.79±2.71 | 21.90±2.77 | 0.353 |
| Basal FSH (IU/L) | 4.13±7.68 | 3.82±2.89 | 0.367 |
| Basal E2 (pg/mL) | 30.42±113.24 | 33.67±210.13 | 0.562 |
| AMH (ng/mL) | 2.52±2.89 | 2.40±2.16 | 0.366 |
| TSH (mIU/L) | 1.86±0.73 | 4.32±0.53 | 0.000 |
| FT3 (pmol/L) | 4.38±0.45 | 4.42±0.47 | 0.820 |
| FT4 (pmol/L) | 13.52±1.47 | 13.35±1.55 | 0.781 |
| Duration of Gn (days) | 11.26±1.42 | 11.29±1.47 | 0.645 |
| Total Gn dose (IU) | 2,529.06±802.74 | 2,509.23±830.38 | 0.584 |
| No. of oocytes retrieved | 10.02±4.31 | 9.97±4.23 | 0.789 |
| No. of usable embryos (n) | 3.90±2.57 | 3.93±2.65 | 0.856 |
| Endometrial thickness on day of ET (mm) | 10.04±1.74 | 10.03±1.93 | 0.857 |
| Clinical pregnancy rate (%) | 3,268/5,552 (58.86%) | 308/536 (57.46%) | 0.530 |
| Miscarriage rate (%) | 414/3,268 (12.67%) | 48/308 (15.58%) | 0.145 |
| Live birth rate (%) | 2,854/5,552 (51.40%) | 260/536 (48.51%) | 0.200 |
TSH, thyroid-stimulating hormone; BMI, body mass index; FSH, follicle-stimulating hormone; E2, estradiol; AMH, anti-Mullerian hormone; ET, embryo transfer.
Table 4
| Characteristic | TSH ≤4 mIU/L | TSH >4 mIU/L | P value |
|---|---|---|---|
| No. of cycles | 5,813 | 275 | |
| Age (years) | 31.20±4.10 | 31.42±4.08 | 0.377 |
| Duration of infertility (years) | 5.79±3.90 | 5.98±3.43 | 0.414 |
| BMI (kg/m2) | 21.80±2.71 | 21.89±2.90 | 0.581 |
| Basal FSH (IU/L) | 4.12±7.53 | 3.83±2.96 | 0.524 |
| Basal E2 (pg/mL) | 30.23±110.86 | 40.49±289.92 | 0.178 |
| AMH (ng/mL) | 2.51±2.87 | 2.44±2.79 | 0.653 |
| TSH (mIU/L) | 1.94±0.81 | 4.86±2.89 | 0.000 |
| FT3 (pmol/L) | 4.42±0.48 | 4.51±0.41 | 0.998 |
| FT4 (pmol/L) | 13.61±1.49 | 13.45±1.53 | 0.958 |
| Duration of Gn (days) | 11.24±1.41 | 11.35±1.48 | 0.385 |
| Total Gn dose (IU) | 2,525.06±803.18 | 2,573.12±845.71 | 0.327 |
| No. of oocytes retrieved | 10.02±4.30 | 9.84±4.32 | 0.484 |
| No. of usable embryos (n) | 3.91±2.57 | 3.91±2.65 | 0.995 |
| Endometrial thickness on day of ET (mm) | 10.04±1.74 | 10.05±1.95 | 0.986 |
| Clinical pregnancy rate (%) | 3,414/5,813 (58.73%) | 162/275 (58.91%) | 0.953 |
| Miscarriage rate (%) | 435/3,414 (12.74%) | 27/162 (16.67%) | 0.146 |
| Live birth rate (%) | 2,979/5,813 (51.24%) | 135/275 (49.10%) | 0.485 |
TSH, thyroid-stimulating hormone; BMI, body mass index; FSH, follicle-stimulating hormone; E2, estradiol; AMH, anti-Mullerian hormone; ET, embryo transfer.
This showed that when the cutoff value of TSH was 3 mIU/L, there was no difference in the general characteristics of patients with TSH ≤3 mIU/L (5,092 cycles) vs. >3 mIU/L (996 cycles). In addition, women were similar with regards to age, duration of infertility, body mass index (BMI), basal follicle-stimulating hormone (FSH) and estradiol (E2), and anti-Mullerian hormone (AMH) (Table 2). There was no difference in FT3 and FT4 levels, duration of Gn, total Gn dose, number of oocytes retrieved, number of usable embryos, and endometrial thickness on day of embryo transfer. While at the 3 mIU/L threshold of TSH level, the TSH ≤3 mIU/L group had a higher clinical pregnancy rate and live birth rate and a lower miscarriage rate than the TSH >3 mIU/L group, only the live birth rate had a statistically significant difference between the TSH ≤3 and >3 mIU/L groups.
When the cutoff value of TSH was 3.5 mIU/L, there was no difference in the general characteristics of patients with TSH ≤3.5 mIU/L (5,552 cycles) vs. >3.5 mIU/L (536 cycles), and at the threshold, the TSH ≤3.5 mIU/L group had a higher clinical pregnancy rate and live birth rate, and lower miscarriage rate. However, there was no statistical difference among groups (Table 3).
When the cutoff value of TSH was 4 mIU/L, there was no difference in general characteristics of patients with TSH ≤4 mIU/L (5,813 cycles) vs. >4 mIU/L (275 cycles), and at the threshold, the TSH ≤4 mIU/L group had a higher clinical pregnancy rate and live birth rate, and a lower miscarriage rate, with no statistical difference among the groups (Table 4).
We then performed a stratified analysis of the relationship between age, duration of infertility, BMI, serum AMH, basal FSH level, TSH level, and clinical pregnancy outcomes by logical regression. The results indicated logical regression for interactions was not statistically significant between serum TSH level, duration of infertility, BMI, serum AMH, basal FSH level, and clinical pregnancy outcomes (Tables 5-7). However, significant interactions were detected between the age of women among live-birth rate and clinical pregnancy rate (OR 0.943, P=0.000; and OR 0.946, P=0.000, respectively).
Table 5
| Variables | OR | 95.0% CI | P value |
|---|---|---|---|
| Age (years) | 0.943 | 0.925–0.962 | 0.000 |
| Duration of infertility (years) | 0.991 | 0.971–1.011 | 0.367 |
| BMI (kg/m2) | 0.997 | 0.965–1.029 | 0.830 |
| AMH (ng/mL) | 1.022 | 0.994–1.048 | 0.137 |
| Basal FSH (IU/L) | 0.984 | 0.945–1.025 | 0.463 |
| TSH (pmol/L) | 1.010 | 0.952–1.072 | 0.741 |
BMI, body mass index; AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
Table 6
| Variables | OR | 95.0% CI | P value |
|---|---|---|---|
| Age (years) | 0.946 | 0.927–0.966 | 0.000 |
| Duration of infertility (years) | 0.988 | 0.968–1.008 | 0.228 |
| BMI (kg/m2) | 0.999 | 0.968–1.032 | 0.972 |
| AMH (ng/mL) | 1.027 | 0.999–1.056 | 0.061 |
| Basal FSH (IU/L) | 0.964 | 0.925–1.005 | 0.084 |
| TSH (pmol/L) | 1.022 | 0.961–1.088 | 0.484 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
Table 7
| Variables | OR | 95.0% CI | P value |
|---|---|---|---|
| Age (years) | 0.989 | 0.950–1.029 | 0.572 |
| Duration of infertility (years) | 1.021 | 0.995–1.048 | 0.118 |
| BMI (kg/m2) | 1.010 | 0.950–1.074 | 0.745 |
| AMH (ng/mL) | 1.009 | 0.959–1.061 | 0.735 |
| Basal FSH (IU/L) | 0.938 | 0.857–1.026 | 0.163 |
| TSH (pmol/L) | 1.024 | 0.931–1.125 | 0.629 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
Discussion
While the role of TSH in pregnancy outcomes in the case of IVF/ICSI is widely debated, the link between the thyroid and hypothalamic-pituitary-ovarian axis is well established (14). TSH and thyroid hormone (FT3, FT4) act by binding to specific G protein-coupled TSH receptors (TSHRs) and nuclear thyroid hormone receptors (THRs) (15). TSHRs are not only present on the surface of thyroid epithelial cells, but are also found on adipose tissue, fibroblasts, and reproductive tissues, including the ovaries and endometrium (4,14,16). TSH is critical for substance metabolism, folliculogenesis, ovine preimplantation embryo, placental formation, adverse pregnancy outcomes and neurological development (17-19). Hyperthyroidism and hypothyroidism during pregnancy have a well-established detrimental impact on menstrual irregularities (irregular or absent menstrual bleeding) and pregnancy complications, including pregnant and obstetric complications, adverse obstetric outcomes (19), and adverse neurological development of offspring (20).
In this retrospective study, we compared the clinical pregnancy outcomes of first attempts of fresh IVF/ICSI cycles in infertile couples when the cutoff of TSH was 3, 3.5, and 4 mIU/L, and the relationship between TSH level and clinical pregnancy outcomes, before submission to assisted reproductive technology (ART) at the Chongqing Institute of Reproductive and Genetic. There are more than 10,000 fresh IVF cycles every year in our institute, which is the largest reproductive center in southwestern China, and our study utilized the largest sample size to date to analyze whether the TSH threshold before IVF affects the pregnancy outcomes. The main results demonstrated that the live birth rate of infertile couples decreased significantly when TSH levels were below 3 mIU/L, while levels of 3.5 and 4 mIU/L were not of clinical significance in pregnancy outcomes. This may be because raising TSH levels from 3 to 4 mIU/L would result in a nearly fourfold decrease in the number of patients in the higher TSH level group. Differences in the miscarriage rate were not statistically significant, which may be due to the smaller sample size.
The Endocrine Society (TES) guidelines in 2007 recommend optimal preconception levels of TSH be <2.5 mIU/L for women before IVF or pregnancy (21). International guidelines from the Practice Committee of the American Society for Reproductive Medicine (Grade C evidence), state thatif pre-pregnancy TSH levels were between 2.5 and 4 mIU/L, treatment measures should involve monitoring levels and treatment when TSH >4 mIU/L or treating with levothyroxine to maintain TSH <2.5 mIU/L (5). Some researchers observed no significant differences in pregnancy outcomes among women undergoing IVF or IUI with TSH levels at 2.5 mIU/L (22-25). Furthermore, we first detected that the live birth rate had a statistically significant difference when the TSH level was 3 mIU/L, and that the ≤3 mIU/L group had a higher live birth rate than the TSH >3 mIU/L group. Due to the small sample size, there was no statistically significant difference when the TSH level was 3.5 or 4 mIU/L, and no association was detected between TSH levels and clinical pregnancy outcomes in patients undergoing IVF/ICSI through ROC curves and logical regression. Therefore, additional data are needed to define the TSH cutoffs of women undergoing IVF.
Controversy remains regarding the TSH threshold before IVF in pregnancy. Recent studies have indicated the cut-offs are too low and may lead to over diagnosis and unnecessary treatment or even overtreatment. Based on of our data, a TSH threshold of 3 mIU/L would result in a live birth rate that decreases in women with higher levels of TSH. Further research is required investigating infertile women who undergo IVF/ICSI to minimize the potential risks associated with lower live birth higher miscarriages rate.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-79/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-79/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-79/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Chongqing Health Center for Women and Children Ethics Committee (No. 2022-RGI-02), Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fazeli PK, Lee H. Response to the Letter to the Editor: "Higher TSH Levels Within the Normal Range Are Associated With Unexpected Infertility". J Clin Endocrinol Metab 2018;103:3112-3.
- Krassas GE. Thyroid disease and female reproduction. Fertil Steril 2000;74:1063-70. [Crossref] [PubMed]
- Mintziori G, Anagnostis P, Toulis KA, et al. Thyroid diseases and female reproduction. Minerva Med 2012;103:47-62. [PubMed]
- Aghajanova L, Stavreus-Evers A, Lindeberg M, et al. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril 2011;95:230-7, 237.e1-2.
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015;104:545-53. [Crossref] [PubMed]
- The Forth Committee Of Chinese Society Of Reproduction Medicine C M A. Chinese Society for Reproductive Medicine consensus for subclinical hypothyroidism in the infertile female population. Chin J Reprod Contracepctive Medicine consensus for subclinical hypothyroidism in the infertile female population. Chin J Reprod Contracep 2019;39:609-6219.
- Pelliccione F, Lania A, Pizzocaro A, et al. Levothyroxine supplementation on assisted reproduction technology (ART) outcomes in women with subtle hypothyroidism: a retrospective study. Gynecol Endocrinol 2018;34:1053-8. [Crossref] [PubMed]
- Fumarola A, Grani G, Romanzi D, et al. Thyroid function in infertile patients undergoing assisted reproduction. Am J Reprod Immunol 2013;70:336-41. [Crossref] [PubMed]
- Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011;21:1081-125. [Crossref] [PubMed]
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2543-65. [Crossref] [PubMed]
- Zhang J, Li W, Chen QB, et al. Establishment of trimester-specific thyroid stimulating hormone and free thyroxine reference interval in pregnant Chinese women using the Beckman Coulter UniCel DxI 600. Clin Chem Lab Med 2015;53:1409-14. [Crossref] [PubMed]
- Grove-Laugesen D, Aaskov C, Ebbehøj E, et al. Preconceptional thyrotropin level in euthyroid women is inversely associated with the live birth rate in first in vitro fertilization cycle. Acta Obstet Gynecol Scand 2019;98:929-36. [Crossref] [PubMed]
- Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol 2005;106:753-7. [Crossref] [PubMed]
- Mintziori G, Goulis DG. In vitro fertilization/intracytoplasmic insemination and thyroid function: reviewing the evidence. Metabolism 2018;86:44-8. [Crossref] [PubMed]
- Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther 2017;173:135-45. [Crossref] [PubMed]
- Aghajanova L, Lindeberg M, Carlsson IB, et al. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online 2009;18:337-47. [Crossref] [PubMed]
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012;22:1200-35. [Crossref] [PubMed]
- Chen S, Zhou X, Zhu H, et al. Preconception TSH and pregnancy outcomes: a population-based cohort study in 184 611 women. Clin Endocrinol (Oxf) 2017;86:816-24. [Crossref] [PubMed]
- Noventa M, Riva A, Buzzaccarini G, et al. P–680 Thyroid function in euthyroid women during controlled ovarian stimulation (COH): does the TSH fluctuations have an impact on IVF outcomes? Human Reproduction 2021. Available online:
10.1093/humrep/deab130.679 10.1093/humrep/deab130.679 - Hou J, Yu P, Zhu H, et al. The impact of maternal hypothyroidism during pregnancy on neonatal outcomes: a systematic review and meta-analysis. Gynecol Endocrinol 2016;32:9-13. [Crossref] [PubMed]
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010;95:4227-34. [Crossref] [PubMed]
- Endocrine Society. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. Thyroid 2007;17:1159-67. [PubMed]
- Green KA, Werner MD, Franasiak JM, et al. Investigating the optimal preconception TSH range for patients undergoing IVF when controlling for embryo quality. J Assist Reprod Genet 2015;32:1469-76. [Crossref] [PubMed]
- Reh A, Grifo J, Danoff A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil Steril 2010;94:2920-2. [Crossref] [PubMed]
- Unuane D, Velkeniers B, Bravenboer B, et al. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum Reprod 2017;32:915-22. [Crossref] [PubMed]


