
Killer hiding under normal oxygen saturation: a case report about methemoglobinemia
Introduction
In children with congenital heart disease, pulmonary hypertension (PH) remains a problem in the postoperative period following cardiac surgery (1). Inhaled nitric oxide (iNO) is a selective vasodilator acting on the pulmonary vascular bed. Nitric oxide (NO) diffuses into blood vessels, where it is rapidly bound to hemoglobin and inactivated (2). Methemoglobin (MetHb) is produced when hemoglobin is oxidized. This change in oxidation state converts the ferrous (Fe2﹢) to the ferric (Fe3﹢) form, which is unable to bind oxygen and, furthermore, results in a left shift of the oxygen-hemoglobin dissociation curve (3). Methemoglobinemia is a potentially life-threatening condition that can present with nonspecific clinical features. Routine oxygen saturation monitoring does not reflect the actual oxygenation status of patients. This lack of specificity can increase the probability of delayed diagnosis or management. We report a case of a child who developed severe methemoglobinemia during iNO therapy after cardiac surgery and demonstrate the limitations of the routine methods used to assess oxygenation levels. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-588/rc) and with written authorization of the child’s family.
Case presentation
A 2-year-old boy, who had suffered repeated pulmonary infections, was further investigated in our hospital. Transthoracic echocardiography (TTE) revealed coarctation of the aorta (CoA), biventricular hypertrophy with an enlarged left heart, patent ductus arteriosus (PDA) with a bidirectional shunt at the aortopulmonary level, persistent left superior vena cava, moderate tricuspid regurgitation [Vmax =4.7 m/s, pressure gradient (PG) =88 mmHg], and severe PH but with normal left ventricular function (ejection fraction, 70%). The child had no significant personal and family history.
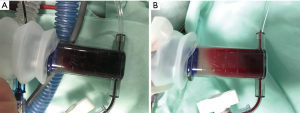
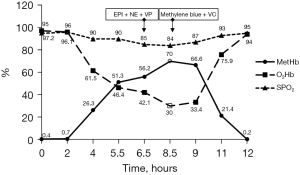
The child underwent correction of aortic coarctation and ligation of the ductus arteriosus without cardiopulmonary bypass. After the operation, he was transferred to the pediatric intensive care unit (PICU) under stable hemodynamic conditions. On postoperative day 1, the child had recurrent hypotension and hypoxemia. An echocardiogram at the bedside showed tricuspid regurgitation (Vmax =3.4 m/s, PG =47 mmHg). NO inhalation was started at 20 ppm on a PB840 ventilator, and the oxygenation status of the child did not improve after half an hour. Therefore, we adjusted the dose to 40 ppm, and the child’s condition gradually improved. Over the course of the next few hours, the oxygenation started to worsen again and SaO2 measured by a pulse oximeter reached a plateau of 83–88% saturation when FiO2 was maintained at 0.5. However, the SaO2 in arterial blood gas (ABG) analysis from the Cobas b 123 poc system (SaO2 is calculated) was 95%, and the hemodynamics were still stable. After confirmation of the endotracheal tube position and changing ventilation setting parameters, this was interpreted as poor pulmonary gas exchange, but MetHb levels were already over 50%. In the next hour, hemodynamics were difficult to maintain. The child appeared visibly cyanosed, and rescue measurements were initiated, including 100% oxygen ventilation, epinephrine, norepinephrine and vasopressin infusion. Repeat echocardiography at the bedside showed improved pulmonary hemodynamics and no other hemodynamic problems, such as pleural effusion or pulmonary edema, and chest X-rays ruled out pneumothorax. The ABG sample was noted as chocolate brown in color (Figure 1A). The following parameters were noted: PO2: 291.4 mmHg, PCO2: 37 mmHg, SaO2: 94%, pH: 7.2, MetHb >70%, and oxyhemoglobin (O2Hb) <30%. Other possible causes of methemoglobinemia were excluded, including: nitrates, chlorates, and local anesthetic. The inciting agent, presumably iNO, was immediately discontinued, while methylene blue (2 mg/kg, 1% solution, i.v.) and vitamin C (VC; 30 mg/kg, i.v.) were immediately administered. Sildenafil was also taken orally (0.5 mg/kg every 6 h) as an alternative treatment. Since this was an emergency, we tested the patient’s glucose-6-phosphate dehydrogenase (G6PD) levels and administered methylene blue before the results returned. Acidosis was actively corrected at the same time. After that, the G6PD, lactate dehydrogenase (LDH), haptoglobulin and total bilirubin levels were normal. The MetHb level fell to 66.6% within 60 min and to 2% at 4 h. After 4 h, the child had good oxygenation (SpO2 95%, FiO2 60%) with normal pink complexion, and hemodynamics became stable with little norepinephrine (0.03 µg/kg/min) infusion. A bright red arterial blood sample was obtained after methylene blue and VC infusion (Figure 1B). ABG showed a drop in MetHb to 0.2%, and the patient’s condition markedly improved with MetHb levels steadily decreasing (Figure 2). Due to recurrent PH and pneumonia, the child was discharged after 4 months of treatment and rehabilitation with normal growth and development until now. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
It is feasible and safe for children with congenital heart disease to receive iNO in the 5–40 ppm range after cardiac surgery (2,4). Methemoglobinemia is a known potential complication of iNO. According to a previous study (4), 10.4% of infants treated with iNO developed methemoglobinemia. Despite the increased use of iNO over the past several years, there have been few cases of life-threatening methemoglobinemia reported in patients induced by iNO (5).
Normal levels of MetHb are less than 1%. The severity of a patient’s symptoms depends on the MetHb level. Cyanosis becomes apparent at approximately 10% concentration (3). Hypoxia does not improve with the administration of oxygen. Fatigue, confusion, nausea, vomiting, tachypnea, and tachycardia occur at concentrations ranging from 30% to 50% (6). Symptoms may be worse if the patient has underlying cardiac or pulmonary disease (7). This child had the confounding factor of severe PH that mimicked methemoglobinemia in many clinical manifestations. At this stage, attention was drawn to the disease itself. Another risk factor for increased MetHb levels is associated with a higher initial iNO concentration; however, the iNO concentration gradually increased in our case. Moreover, the child was sedated, paralyzed, and ventilated, which can increase the probability of misdiagnosis.
Barker et al. (8) used animal models to show that SpO2 initially decreases with increasing MetHb%. When MetHb% reaches 30–35%, SpO2 reaches a plateau in the 82–86% range and then becomes virtually independent of MetHb%. This trend toward 85% is noted in our case. Traditional pulse oximetry measures light absorbance at 2 distinct wavelengths (660 and 940 nm) and determines the O2Hb/deoxyhemoglobin (HHb) ratio at 2 different wavelengths (9). The presence of MetHb increases both the numerator and denominator of this ratio, which tends to drive the ratio toward 1 and results in a saturation reading of 85% (10). Thus, during the initial evaluation of oxygen status in the presence of dyshemoglobins, it is important not to rely only on pulse oximetry. Furthermore, ABG provides another way to assess oxygenation status. Unfortunately, this approach of calculating SaO2 assumes a normal oxygen dissociation curve with no presence of inherited or acquired dyshaemoglobins (10). Currently, fractional oxyhemoglobin (FO2Hb) is the most reliable indicator of a patient’s true oxygen saturation. Barker et al. (8) demonstrates that FO2Hb decreases linearly with increasing MetHb%, and the data lie near the line FO2Hb = 100 − MetHb% (linear regression slope = −0.996; intercept =99.6%). Therefore, when the MetHb level in our children’s blood was greater than 70% and SaO2 was maintained at 95%, but O2Hb was lower than 30% which correlated to the child’s extremely hypoxic state. In contrast to a traditional pulse oximeter, CO-oximeters may report the various hemoglobin fractions that can be used to diagnose multiple hemoglobinopathies, including both carboxy-Hb and MethHb (3). Although Co-oximeter is not available in our facility, it is recommended as a noninvasive and reliable monitoring method for oxygen saturation in patients treated with NO.
First-line treatment in patients with methemoglobinemia is supportive therapy and discontinuation of the offending agent. Methylene blue 1% and 1 to 2 mg/kg intravenously over 5 min can be administered to correct methemoglobinemia (6). However, it is ineffective in patients with G6PD deficiency and has the potential to induce hemolytic anemia (3). For this patient, the serum G6PD level was normal, and there was no active hemolysis. Successful treatment of methemoglobinemia with VC has been reported previously (11,12). Low-dose VC was used in this case as an adjuvant antidote because of concerns that high doses of VC causing renal failure related to hyperoxaluria (12). Additionally, when methylene blue is ineffective or contraindications exist, exchange transfusions and hyperbaric oxygen therapy can be considered (7,13). However, there is no clinical research indicating that these treatments are useful.
The monitoring and management of MetHb during iNO therapy is important. First, delivery of the gas close to the patient affords less dead space in which NO may accumulate (14). In addition, NO delivery systems should provide constant concentrations of NO independent of ventilator mode or settings, ensure rapid mixing and minimize the exposure time of NO to oxygen. It has been reported that MetHb monitoring in pediatric patients receiving iNO therapy should be based on clinical evidence (15). We still recommend routine, ongoing monitoring of MetHb levels in all children treated with 40 ppm iNO, especially in the presence of comorbidities that may predispose subjects to methemoglobinemia.
This case highlights the possibility of developing life-threatening methemoglobinemia during standard iNO therapy. In these patients, SpO2 or calculated oxygen saturation in ABG is a “hidden killer”, normal value but without reflecting real arterial oxygen content or tissue oxygen delivery, and O2Hb in the blood should be focused on. Low doses of VC can be used as a safe adjuvant antidote to reduce the risks associated with high doses of methylene blue.
Acknowledgments
We appreciate the contributions of all the surgeons involved.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-588/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-588/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-588/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Day RW, Hawkins JA, McGough EC, et al. Randomized controlled study of inhaled nitric oxide after operation for congenital heart disease. Ann Thorac Surg 2000;69:1907-12; discussion 1913. [Crossref] [PubMed]
- Hermon MM, Burda G, Golej J, et al. Methemoglobin formation in children with congenital heart disease treated with inhaled nitric oxide after cardiac surgery. Intensive Care Med 2003;29:447-52. [Crossref] [PubMed]
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014;28:1043-7. [Crossref] [PubMed]
- Hamon I, Gauthier-Moulinier H, Grelet-Dessioux E, et al. Methaemoglobinaemia risk factors with inhaled nitric oxide therapy in newborn infants. Acta Paediatr 2010;99:1467-73. [Crossref] [PubMed]
- Centorrino R, Shankar-Aguilera S, Foligno S, et al. Life-Threatening Extreme Methemoglobinemia during Standard Dose Nitric Oxide Therapy. Neonatology 2019;116:295-8. [Crossref] [PubMed]
- Cefalu JN, Joshi TV, Spalitta MJ, et al. Methemoglobinemia in the Operating Room and Intensive Care Unit: Early Recognition, Pathophysiology, and Management. Adv Ther 2020;37:1714-23. [Crossref] [PubMed]
- Syed AU, Jelly AE, Algebaly AA, et al. Methemoglobinemia due to nitric oxide therapy in a child after cardiac surgery. Asian Cardiovasc Thorac Ann 2013;21:345-7. [Crossref] [PubMed]
- Barker SJ, Tremper KK, Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology 1989;70:112-7. [Crossref] [PubMed]
- Sinex JE. Pulse oximetry: principles and limitations. Am J Emerg Med 1999;17:59-67. [Crossref] [PubMed]
- Haymond S, Cariappa R, Eby CS, et al. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005;51:434-44. [Crossref] [PubMed]
- Dhibar DP, Sahu KK, Jain S, et al. Methemoglobinemia in a Case of Paint Thinner Intoxication, Treated Successfully with Vitamin C. J Emerg Med 2018;54:221-4. [Crossref] [PubMed]
- Park EJ, Lee M, Min YG. Successful treatment of NO-induced methemoglobinemia with low-dose vitamin C. Clin Toxicol (Phila) 2017;55:686. [Crossref] [PubMed]
- Altintop I, Sanri E, Tatli M, et al. Methemoglobinemia treated with hyperbaric oxygen therapy: A case report. Turk J Emerg Med 2018;18:176-8. [Crossref] [PubMed]
- Taylor MB, Christian KG, Patel N, et al. Methemoglobinemia: Toxicity of inhaled nitric oxide therapy. Pediatr Crit Care Med 2001;2:99-101. [Crossref] [PubMed]
- Thomas CA, Valentine K. Utility of routine methemoglobin laboratory assays in critically ill pediatric subjects receiving inhaled nitric oxide. J Crit Care 2018;48:63-5. [Crossref] [PubMed]


