
Fingolimod protects against experimental necrotizing enterocolitis by regulating intestinal T cell differentiation
Introduction
Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality from gastrointestinal disease in premature infants, affecting about 5–10% of all very low birth weight infants (1). Although advances have been made in neonatal intensive care, the total number of infants affected by NEC has remained unchanged or increased slowly, partly due to the increased number of preterm births (2). Survivors of NEC usually experience lifelong sequelae, such as short bowel syndrome and impaired neurodevelopment. Thus, NEC presents both an emotional and financial burden on families and society.
Multiple studies have suggested that excessive immune responses contribute to NEC pathology (3,4). Previous research has mainly focused on the complex network of intestinal epithelium and the innate immune system including the physical barrier of the intestine, expression of toll-like receptor 4 (TLR4), and innate lymphoid cells, which may be altered during the early stages of the inflammatory response (5). However, inhibition of the early inflammatory cascade appears to have limited benefits. As a result, an increasing number of studies have focused on proinflammatory effector immune cells, which are downstream of the early inflammatory response during NEC development (6,7). One study has found increased T cell infiltration into the small intestines of infants with NEC, and significant changes in the proportion of effector T cells and regulatory T (Treg) cells in the inflamed intestine (6). Overexpression of TLR4 in intestinal epithelial cells can promote T cell infiltration and affect T cell differentiation (6). Treatment strategies targeting adaptive immunity are considered an important approach. Retinoic acid, commensal Propionibacterium, and melatonin have been shown to correct T helper 17 (Th17)/Treg cell imbalance in intestinal tissues and exert protective effects on NEC (6-8). These studies have demonstrated that abnormal migration and differentiation of T cells play an important role in the pathogenesis of NEC and represent potential therapeutic targets.
Fingolimod (FTY720) is an immunosuppressive agent that is mainly used for the treatment of multiple sclerosis (9). As it has significant effects without inducing general immunosuppression, FTY720 can be used to treat a variety of inflammatory diseases. It exerts immunosuppressive effects by regulating the migration of T cells, and has been shown to be effective in preventing transplant rejection and treating autoimmune diseases (10). In addition, FTY720 can improve diarrhea caused by inflammatory bowel disease, reduce intestinal inflammation, and increase weight (11). Dominguez-Villar et al. found that FTY720 can also directly modulate Treg phenotype from interferon-γ (IFN-γ)-positive to interleukin (IL)-10-positive, as well as decrease the number of interleukin IL-17-positive Th17 cells in MS patients (12). However, treating activated T cells with FTY720 in vitro did not inhibit the expression of IL-17. Thus, the precise mechanism of action of FTY720 requires further elucidation.
In NEC, it remains unclear whether FTY720 affects macrophages and T cells. Thus, we explored the regulatory effect of FTY720 on immune cells in intestinal tissues of NEC mice in vivo. We also conducted in vitro experiments to determine the possible mechanism of action of FTY720, and further examined the inhibition of microglial activation by FTY720 in brain tissues of NEC mice. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-92/rc).
Methods
Experimental NEC model
All animal experiments were approved by the Animal Ethics and Welfare Committee of Shanghai Children’s Hospital (No. 2018RY027-E01), in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. A protocol was prepared before the study without registration. We separated 5-day-old C57BL/6 neonatal male/female mice (Shanghai JieSiJie Laboratory Co. Ltd., Shanghai, China) from their mothers and placed them in neonatal incubators at a temperature of 32 °C and a humidity of 40%. We induced NEC in neonatal mice by gavage feeding of hyperosmolar formula (15 g Similac dissolved in 75 mL Esbilac), exposure to hypoxia (5% O2 for 10 min, 3 times daily), and oral lipopolysaccharide (LPS; #L2630, Sigma-Aldrich, St. Louis, MO, USA) at a dosage of 4 mg/kg/day as previously described (13). In the NEC + FTY720 group, pups were treated with oral gavage of FTY720 (1 µg/g; #SML0700, Sigma-Aldrich) every day in addition to NEC induction. Breastfed mice without exposure to cold or hypoxic stress served as controls. All surviving pups were euthanized 96 hours after NEC induction and their ilea were collected for further analysis.
Histological examination
Ileal tissue (about 2 cm away from the ileocecal valve) was collected, fixed with formalin, embedded in paraffin, sectioned at a thickness of 5 µm, and stained with hematoxylin and eosin (H&E). Stained sections (n=10 for NEC, NEC + FTY720, and control group) were assessed by 3 blinded investigators using an established NEC histopathological scoring system (14).
Quantitative messenger RNA expression analysis
We isolated RNA from intestinal and cultured cell samples using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Purified RNA was quantified by NanoDrop™ Spectrophotometers (Thermo Fisher Scientific, Waltham, MA, USA) and 1 µg of RNA was used for complementary DNA (DNA) synthesis (qScript cDNA SuperMix, QuantaBio, Beverly, MA, USA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR™ Green Master Mix (Wisent, Saint-Bruno, QC, Canada). In total, 40 cycles were performed under the following conditions: denaturation at 95 ℃, annealing at 58 ℃, and extending at 72 ℃, and using the following primer sequences: Rorc (forward: 5'-ACAAATTGAAGTGATCCCTTGC-3', reverse: 5'-GGAGTAGGCCACATTACACTG-3'); FOXP3 (forward: 5'-TTTCACCTATGCCACCCTTATC-3', reverse: 5'-CATGCGAGTAAACCAATGGTAG-3'); Arg1 (forward: 5'-ATTCCCTCACCCTGTATGCC-3', reverse: 5'-CTCTGGTCCACAATCCCGAAC-3'); IL-6 (forward: 5'-CTCCCAACAGACCTGTCTATAC-3', reverse: 5'-CCATTGCACAACTCTTTTCTCA-3'); IL-23 (forward: 5'-AATAATGTGCCCCGTATCCAG-3', reverse: 5'-GCTCCCCTTTGAAGATGTCAG-3'); Mrc1 (forward: 5'-CTTCCGTCACCCTGTATGCC-3', reverse: 5'-ATCTGCTCCACAATCCCGAAC-3'); iNOS (forward: 5'-GTTCTCAGCCCAACAATACAAGA-3', reverse: 5'-GTGGACGGGTCGATGTCAC-3'); IL1-β (forward: 5'-CGCAGCAGCACATCAACAAGAGC-3', reverse: 5'-TGTCCTCATCCTGGAAGGTCCACG-3'); and Tnf-α (forward: 5'-CTTCAGGGATATGTGATGGACTC-3', reverse: 5'-GGAGACCTCTGGGGAGATGT-3'). The expression levels of other genes were calculated by the 2−∆∆Ct method, and normalized to the reference housekeeping gene β-actin.
Preparation of peritoneal macrophages (PMs) and naïve T-cells
PMs were prepared as follows: 1 mL of 3.8% thioglycolate medium (# TM4635, Thermo Fisher) was injected into the peritoneal cavity of each C57BL/6 mouse. After 3 days, mice were anesthetized with CO2 and euthanized by extracting their eyeballs to let them bleed out. This technique reduced the risk of bleeding into the abdominal cavity. Next, the abdomen was cleaned with 70% ethanol. Using ophthalmic scissors, a lateral incision was made along the midline of the bottom of the peritoneum. The peritoneal cavity was then injected with 5 mL of ice-cold Dulbecco’s phosphate-buffered saline (PBS), and after 30 minutes, the peritoneal fluid was recovered. Peritoneal cells were then centrifuged for 10 minutes at 400 ×g in a refrigerated centrifuge. Cells were counted using a hemocytometer and the cell density was adjusted to 1×106 cells/mL. The cell suspension was placed into a 12-well plate and peritoneal cells were cultured in Dulbecco’s modified eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) at 37 ℃ with 5% CO2 and saturated humidity for 2 hours. Then, the supernatant was discarded, the wells were washed with DMEM containing no FBS to remove non-adherent cells and obtain the adherent cells (macrophages).
Purified T cells were isolated from the spleen of uninfected mice by magnetic cell sorting using the CD4+ T Cell Isolation Kit (#130-117-043, Miltenyi Biotec, Bergish Gladbach, Germany). The purity of the T cells was assessed by flow cytometry.
Reagents and co-culturing conditions
Cells were divided into four groups: (I) control (untreated); (II) LPS (1 µM); (III) LPS + FTY720 (400 nM); and (IV) LPS + FTY720 (1,500 nM). The purified CD4+ T cells (1×106 cells/well) were activated with anti-CD3 and anti-CD28 antibodies (#clone145-2C11, #clone 37.51, BioLegend, San Diego, CA, USA) for 3 days, then co-cultured with LPS-stimulated or FTY720 + LPS co-treated PMs (1×105 cells/well) in DMEM containing 10% FBS for 5 days at 37 ℃ with 5% CO2. The cell culture supernatant was collected, and cytokines were detected by enzyme-linked immunosorbent assay (ELISA).
Western blot
Samples were minced and sonicated for 5 minutes in ice-cold radioimmunoprecipitation assay (RIPA) buffer [#8553S, Cell Signaling Technology (CST), Danvers, MA, USA]. The lysate (30 µg) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (ISEQ. 00010; Millipore, Billerica, MA, USA). Afterwards, the membrane was incubated with blocking buffer [tris-buffered saline containing 0.1% Tween-20 (TBST) and 5% bovine serum albumin (BSA)] at room temperature for 1 hour, incubated with primary antibody occluding (#ab167161, Abcam, Cambridge, MA, USA) overnight at 4 ℃, and then incubated with secondary antibody for 1 hour at room temperature. The result of western blotting was detected by ImmobilonTM Western Chemiluminescent HRP Substrate (#WBKLS0500, Millipore).
ELISA
The IL-17 levels in the culture supernatant were measured with an ELISA kit (#ab100702, Abcam) according to the manufacturer’s protocol.
Immunofluorescent and immunohistochemical staining
Paraffin-embedded tissues were sectioned at a thickness of 3 µm, followed by routine dewaxing and hydration. Tissue sections were incubated for 10 minutes in 3% hydrogen peroxide after antigen retrieval. Next, the sections were permeabilized and blocked with 3% BSA containing 0.1% Triton X-100 for 1 hour at room temperature. Tissue samples were then incubated with the primary antibodies against CD3 (#ab135372, Abcam) and Iba-1 (#ab178846, Abcam) overnight at 4 ℃ in a humidity chamber. Finally, samples were incubated with Alexa Fluor® (Thermo Fisher, USA) conjugated secondary antibodies at room temperature for 1 hour before being rinsed with distilled water.
Statistical methods
Data were expressed as mean ± standard deviation. Student’s t-test was performed to determine differences between two groups. Analysis of variance (ANOVA) was used for multiple group comparisons. A P value <0.05 was considered statistically significant. Kaplan-Meier survival curves were generated and compared using the log-rank test. All cell experiments were repeated 3 times.
Results
FTY720 improves survival and ameliorates intestinal inflammation in experimental NEC
Survival rate is an important indicator of disease severity in NEC animal models. We found that the 4-day survival rate of neonatal mice receiving hyperosmolar formula and LPS with exposure to hypoxia was 30%. Oral administration of FTY720 significantly improved the survival rate (Figure 1A). We also found that NEC mice had intestinal epithelial damage, villous sloughing, and transmural inflammatory infiltrate, while FTY720 treatment improved histological damage of the intestines (Figure 1B,1C). Tight junction plays a protective role in intestinal tissue. We found that occludin was decreased in NEC mice and was reversed by addition of FTY720 (Figure 1D). The inflammation in NEC is characterized by the upregulation of inducible nitric oxide synthase (iNOS). Our findings are consistent with one previous research, however treatment with FTY720 strongly suppressed messenger RNA (mRNA) expression of iNOS to a level similar to that of the control group (Figure 1E) (15).
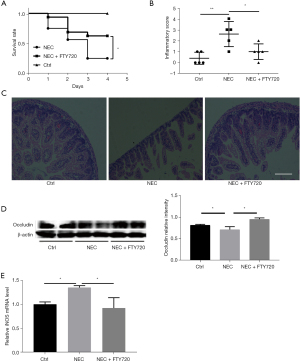
FTY720 improves the Th17/Treg balance in intestinal tissues and reduces microglial activation in experimental NEC
Since T cells play an important role in the development of NEC, we sought to determine whether FTY720 could inhibit T cell infiltration in NEC mice. We found a significant decrease in T cell infiltration in the intestines of NEC mice treated with FTY720 at a dosage of 1 mg/kg (Figure 2A). Next, we performed immunohistochemistry (IHC) staining to measure the expression of IL-17, a characteristic cytokine produced by Th17 cells, in the intestines of NEC mice. We found that IL-17 expression in the NEC group was significantly increased, while the number of Th17 cells in the NEC + FTY720 group was decreased (Figure 2B). We used RT-PCR analysis to measure the expression level of Rorc, the main Th17 cell transcription factor, in the three groups. We found that Rorc expression was significantly increased in the NEC group, while FTY720 treatment led to decreased Rorc expression. In addition, we measured the expression level of Foxp3, the main Treg cell transcription factor, in the three groups. We found significantly decreased expression of Foxp3 in the NEC group, which was increased following treatment with FTY720 (Figure 2C).
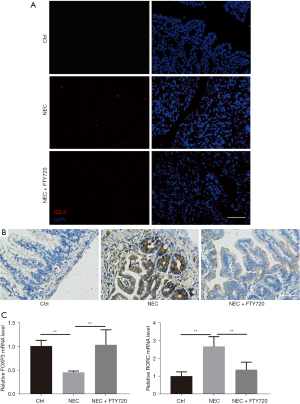
FTY720 regulates macrophage differentiation into the M2b phenotype
Various concentrations of FTY720 had no significant effect on cell viability(Figure 3A). We stimulated PMs isolated from C57BL/6 mice with LPS (1 µM) or LPS + FTY720 (400 nM) for 24 hours and found a significant change in macrophage morphology. The LPS-treated M1 macrophages were flattened into a round pancake-like shape within 24 hours. In contrast, FTY720-treated macrophages had an elongated shape with multiple projecting antennae (Figure 3B). The RT-PCR analysis revealed that FTY720 promoted macrophage polarization into the M2 phenotype. High IL-6 mRNA expression was observed in both the FTY720 and LPS groups. However, mRNA expression of Mannose receptor (Mrc1), markers of the M2 phenotype, was significantly increased in the FTY720 group compared with the LPS group (Figure 3C). Interestingly, arginase-1 (Arg-1) was decreased in FTY720 group and expression of IL-6 mRNA in FTY720 group increased as same as LPS group. All these findings suggest that the co-stimulation of FTY720 and LPS on macrophages induced differentiation into the M2b macrophage phenotype, which is associated with a strong anti-inflammatory response (Figure 3C). In addition, FTY720 inhibited the expression of IL-23, the main cytokine that promotes the Th17 phenotype (Figure 3C).
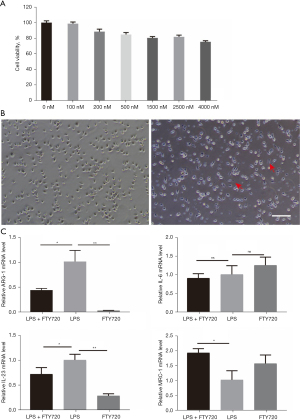
FTY720 affects T cell differentiation indirectly through macrophages
The purity of CD4+ T cells separated from the mouse spleen by magnetic-activated cell sorting was above 87.83% (Figure 4A). Cell morphology could be an indicator of macrophage activation status. We also showed that macrophages exhibited elongated shape in co-culture system (Figure 4B). To determine whether FTY720 could directly induce differentiation of naive T cells, we activated T cells with anti-CD3 and anti-CD28 antibodies and treated them with FTY720 3 days later. Our ELISA data revealed no significant changes in IL-17 levels in the culture supernatant after FTY720 treatment (Figure 4C).
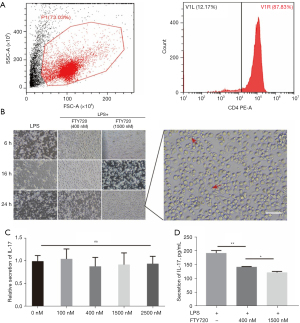
However, significant differences in IL-17 levels in the culture supernatant of macrophages treated with LPS or LPS + FTY720 and cultured with T cells for 5 days (macrophages were washed with PBS 3 times before culturing) were observed. The M1-polarized macrophages could effectively stimulate the differentiation of naive T cells into Th17 cells, while FTY720 could significantly inhibit such differentiation (Figure 4D).
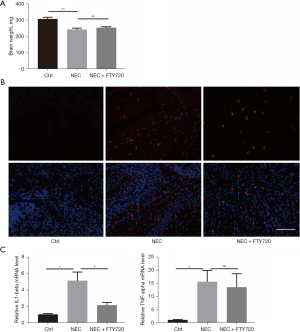
FTY720 decrease the inflammation in NEC associated brain injury
Recently, NEC associated neuro-inflammation has been attracting more and more attention from global researchers. Therefore, it is urgent to understand the effects of FTY720 on NEC related brain injury. There was a significant brain weight loss between the NEC and healthy control groups, while FTY720 treatments failed to protect (Figure 5A). The increased expression of microglial marker Iba-1 is associated with increased inflammation of the nervous system. Since one previous study has shown microglial activation in NEC mice, we explored the role of FTY720 in the activation of microglia in the nervous system (16). Immunofluorescence staining showed that FTY720 can inhibit the expression of Iba-1 and the occurrence of inflammation (Figure 5B). Furthermore, the IL-1β mRNA—a key pro-inflammatory mediator—was increased in brain of NEC mice, whereas FTY720 treatment abrogated this increase. Induction of NEC enhanced the tumor necrosis factor-alpha expression in the brain and FTY720 failed to decrease its expression (Figure 5C).
Discussion
Perturbation of the gut microbiome and excessive inflammatory responses are associated with the development of NEC in preterm infants (4). Low gestational age and low birth weight are the most frequently reported prognostic factors for neonatal NEC in the literature. These factors may be indicative of a more immaturely developed intestinal system, suggesting a defect in inflammatory regulation, which leads to an irreversible inflammatory cascade. In addition, formula feeding is also recognized as a risk factor for the development of NEC. Breastfeeding can compensate for the immaturity of the gastrointestinal tract and immune system in premature infants in various ways, for example, by neutralizing stomach acid, enhancing intestinal motility and reducing epithelial damage (17). Breastfeeding is currently considered the safest and most effective preventive and therapeutic measure. Moreover, probiotic bacteria have been associated with decreased risk of NEC.
New therapeutic strategies are urgently required to reduce the high morbidity and mortality associated with NEC. Immune cells are involved in the pathophysiological process of intestinal injury from the onset to subsequent development of NEC. The immunosuppressant FTY720 is potent, has few side effects, and accumulating evidence has confirmed its efficacy in treating inflammatory diseases. FTY720 exhibits functional antagonism as a high affinity agonist of the S1P receptor (9). The use of FTY720 to inhibit S1P1 receptor activation has emerged as a major treatment for multiple sclerosis (9). In addition to receptor-dependent activity, recent studies have suggested that FTY720 may also regulate cellular activity independent of receptor activation. These “off-target” effects include activation of intracellular signaling pathways, and epigenetic regulation of transcription (18,19). Additionally, FTY720 has been shown to exert beneficial effects by inhibiting the autophagy pathway by decreasing induction of autophagosome proteins, such as microtubule-associated protein 1 light chain 3 (LC-3-II) and Beclin 1 (20). Study has found that FTY720 has a direct effect on the ratio of Th17/Treg cells in MS patients (12). This clinical phenomenon requires further elucidation as to how FTY720 modulates differentiation of T cells. Herein, we examined the effect of FTY720 on mice subjected to experimental NEC in vivo, as well as on purified T cells following macrophage stimulation in vitro. Our results demonstrated that FTY720 pretreatment protected cells from experimental NEC-induced intestinal injury in vivo and from overexpression of IL-17 in vitro.
Our study indicated that NEC increased the mortality of mice, as well as aggravated histological intestinal damage as shown by an increasing inflammation score. Administration of FTY720 significantly elevated the survival rate and ameliorated pathological injury in NEC mice, which is consistent with previous study (21). However, the precise mechanism of action of FTY720 requires further elucidation.
Harmful stimulations such as hypoxia, infection, oxidative stress, and toxic metabolites disrupt ER homeostasis and lead to ER dysfunction. Under these conditions, proteins are unable to fold properly, which leads to induction of endoplasmic reticulum stress (ERS). Humans and mice with necrotizing enterocolitis (NEC) exhibit higher ER stress and apoptosis in the crypt, whereas genetic inhibition of the stress-related proteins PERK or CHOP attenuates ER stress and NEC severity (22). A variety of therapeutic measures have been developed for ERS, such as fish oil, TUDCA and amniotic fluid stem cells (23-25). All of these measures have been shown to play a beneficial role in animal models of NEC. Intestinal stem cell ER stress and the unfolded protein response can also modulate Th17 cell function through overexpression of CHOP in developing Th17 cells resulting in the inhibition of IL-17A production (26). Therefore, T cell differentiation may be the downstream end point of multiple signaling pathways, although this requires further investigation. The influx of T cells into intestinal tissues is a key step in the exacerbation of NEC. Neonatal infants with NEC have a lower proportion of Tregs in the intestinal lamina propria (27). Egan et al. have previously shown that a large number of CD4+ T cells infiltrate into the intestine of NEC infants, and that mice without functional T cells are protected from NEC development (6). At present, several therapeutic regimens have been used to treat NEC animal models with Th17/Treg imbalance. Melatonin can improve NEC by activating the AMPK/SIRT1 pathway to prevent Th17/Treg imbalance (28). In addition, all-trans retinoic acid (ATRA) has been shown to induce Treg populations and restrict CD4+ Th17 cells (6). The propionibacterium strain, P. UF1 reportedly controls polarization of proinflammatory cells towards a regulatory cell phenotype that may resist disease progression (8). Thus, these studies together with our findings suggest that targeting the Th17/Treg balance is a promising potential treatment strategy for NEC.
As a well-established inhibitor, FTY720 impedes lymphocyte egress from the lymph nodes (29). Here, we found that FTY720 did indeed reduce the infiltration of T cells into the intestines of NEC mice. Furthermore, IL-17-positive cells were increased in the intestinal mucosa of NEC mice, which was blocked by FTY720 treatment. It remains to be determined whether the reduction in T cells is due to the inhibitory effect of FTY720 on migration, or whether the change in T cell numbers is due to a change in phenotype. The major transcription factor of Treg, FOXP3, was dramatically increased in FTY720-treated mice, suggesting that FTY720 might not only suppress T cell migration but also have an effect on T cell differentiation.
Thus, we sought to determine the precise mechanism by which FTY720 mediates changes in T cell phenotypes. Consistent with previous study, we found that treatment of activated T cells with FTY720 had no effect on the secretion of IL-17 in vitro (10). Although many studies have suggested that dendritic cells (DCs) are the main drivers of CD4+ T helper cell polarization, there is also evidence that macrophages may play a role in this process (30-32). Increasing evidence points to an association between the differentiation of T cells and macrophages. The IL-10 produced by macrophages is critical for FOXP3+ Treg cell development, and macrophages are indispensable in Th17-dominated forms of inflammation (33). Therefore, we examined whether FTY720 could decrease the production of IL-17 through modulating M2 polarization. Our data revealed an increased percentage of M2 macrophages following stimulation with LPS and FTY720 in vitro. Furthermore, we found that FTY720 changed the cell morphology and increased expression of the M2b biomarkers IL-6, ARG-1, and MRC-1 in vitro. Thus, our study demonstrates for the first time that FTY720 modulates Th17 cells through macrophage differentiation.
Th17 and Treg cells antagonize each other in function and differentiation. Various factors, epigenetic modifications, metabolic pathways, and microbiota have been shown to modulate plasticity between Treg and Th17 cells. TGF-β and IL-6 are required for the differentiation of naive CD4+ T cells into Th17 cells (34,35). TGF-β induces expression of the retinoic acid-related orphan receptor RORγt, a master regulator of the Th17 lineage. However, despite its induction of RORγt, TGF-β alone cannot initiate Th17 differentiation. TGF-β can also induce the expression of Foxp3, which is the most important transcription factor of Treg cells. Foxp3 inhibits RORγt-directed IL-17A expression by binding to RORγt (36). In the presence of IL-6, the transcription factor STAT3 is activated and can inhibit the expression of Foxp3, which leads to increased levels of RORγt and increased differentiation of Th17 cells (37). High expression of TGFβ induces Foxp3 expression, thereby promoting Treg differentiation, while low levels of TGFβ together with IL-6 induce Th17 polarization. When activated macrophages polarize towards the M1 phenotype, their characteristic cytokine IL-6 is secreted in abundance, thereby polarizing naive T cells towards the Th17 phenotype. When polarized to the M2 phenotype, macrophages secrete large amounts of TGF-β, which induce naive T cells to differentiate into Treg cells. During intestinal inflammation associated with NEC, increased expression of iNOS and IL-6 and decreased expression of TGFβ, are favorable for Th17 cell development. In addition, IL-23 is responsible for the expansion and stabilization of Th17 cells (38). In a T-cell transfer model of experimental allergic encephalomyelitis (EAE), Th17 cells expanded and induced disease only in the presence of IL-23 (39). Our results showed that FTY720 did not affect IL-6 expression in macrophages, but did significantly inhibit IL-23 expression, suggesting that FTY720 may affect the function of Th17 cells through IL-23. Monocytes from NEC patients exhibited a proinflammatory phenotype, which might induce secretion of proinflammatory cytokines (40). Thus, a therapeutic approach might be to inhibit inflammatory monocytes. Moreover, research on experimental NEC has suggested that FTY720 suppresses the NEC-activated CXCL5/CXCR2 axis (21). Previous study has shown that FTY720 can inhibit the function of bone marrow-derived DCs (41). We found that FTY720 can induce macrophages to differentiate into M2b macrophages. Due to its strong anti-inflammatory capacity, in extreme conditions, the over-inhibition of pro-inflammatory cells may even promote bacterial infection (14), which may be an alternative effect of FTY720 on NEC mice.
Neurodevelopmental impairment frequently occurs as a long-term consequence in patients who survive NEC (4). A recent study revealed that reducing microglial activation might protect patients from the development of cognitive impairments (16). Being fat-soluble, FTY720 can pass through the blood-brain barrier easily. In our study, FTY720 downregulated the activation of microglia, and exhibited a potential therapeutic effect on NEC-associated brain injury.
In conclusion, we found that FTY720 can improve the survival rate and degree of intestinal damage in NEC animal models. It can restore intestinal balance by inhibiting the infiltration of T cells in inflamed intestinal tissues and altering naive T cell differentiation. In addition, we identified the necessary factors (namely the macrophage) required for FTY720-dependent regulation of naive T cell differentiation through cell co-culture. We also found that FTY720 treatment can reduce NEC-related brain inflammation. However, the therapeutic effect of FTY720 on NEC-associated neuro-impairment needs further investigation in the light of lifelong sequelae in NEC brain injury.
Acknowledgments
Funding: This work was supported by Natural Science Foundation (Nos. 82001591 and 82171696).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-92/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-92/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-92/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the Animal Ethics and Welfare Committee of Shanghai Children’s Hospital (No. 2018RY027-E01), in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44:1072-5; discussion 1075-6. [Crossref] [PubMed]
- Thyoka M, de Coppi P, Eaton S, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg 2012;22:8-12. [Crossref] [PubMed]
- Mara MA, Good M, Weitkamp JH. Innate and adaptive immunity in necrotizing enterocolitis. Semin Fetal Neonatal Med 2018;23:394-9. [Crossref] [PubMed]
- Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016;13:590-600. [Crossref] [PubMed]
- Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015;3:e1000707. [Crossref] [PubMed]
- Egan CE, Sodhi CP, Good M, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 2016;126:495-508. [Crossref] [PubMed]
- Gao X, Liu W, Gao P, et al. Melatonin-induced lncRNA LINC01512 prevents Treg/Th17 imbalance by promoting SIRT1 expression in necrotizing enterocolitis. Int Immunopharmacol 2021;96:107787. [Crossref] [PubMed]
- Colliou N, Ge Y, Sahay B, et al. Commensal Propionibacterium strain UF1 mitigates intestinal inflammation via Th17 cell regulation. J Clin Invest 2017;127:3970-86. [Crossref] [PubMed]
- Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 2010;9:883-97. [Crossref] [PubMed]
- Mehling M, Lindberg R, Raulf F, et al. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology 2010;75:403-10. [Crossref] [PubMed]
- Daniel C, Sartory N, Zahn N, et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol 2007;178:2458-68. [Crossref] [PubMed]
- Dominguez-Villar M, Raddassi K, Danielsen AC, et al. Fingolimod modulates T cell phenotype and regulatory T cell plasticity in vivo. J Autoimmun 2019;96:40-9. [Crossref] [PubMed]
- Chen Y, Koike Y, Miyake H, et al. Formula feeding and systemic hypoxia synergistically induce intestinal hypoxia in experimental necrotizing enterocolitis. Pediatr Surg Int 2016;32:1115-9. [Crossref] [PubMed]
- Nishiguchi T, Ito I, Lee JO, et al. Macrophage polarization and MRSA infection in burned mice. Immunol Cell Biol 2017;95:198-206. [Crossref] [PubMed]
- Sun RZ, Fan Y, Liang X, et al. Rapamycin and FTY720 Alleviate Atherosclerosis by Cross Talk of Macrophage Polarization and Autophagy. Biomed Res Int 2018;2018:1010248. [Crossref] [PubMed]
- Niño DF, Zhou Q, Yamaguchi Y, et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med 2018;10:eaan0237. [Crossref] [PubMed]
- Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis! Semin Perinatol 2017;41:36-40. [Crossref] [PubMed]
- Gardner NM, Riley RT, Showker JL, et al. Elevated Nuclear and Cytoplasmic FTY720-Phosphate in Mouse Embryonic Fibroblasts Suggests the Potential for Multiple Mechanisms in FTY720-Induced Neural Tube Defects. Toxicol Sci 2016;150:161-8. [Crossref] [PubMed]
- Ji J, Wang J, Yang J, et al. The Intra-nuclear SphK2-S1P Axis Facilitates M1-to-M2 Shift of Microglia via Suppressing HDAC1-Mediated KLF4 Deacetylation. Front Immunol 2019;10:1241. [Crossref] [PubMed]
- Li X, Wang MH, Qin C, et al. Fingolimod suppresses neuronal autophagy through the mTOR/p70S6K pathway and alleviates ischemic brain damage in mice. PLoS One 2017;12:e0188748. [Crossref] [PubMed]
- Feng Z, Zhou H, Ma S, et al. FTY720 attenuates intestinal injury and suppresses inflammation in experimental necrotizing enterocolitis via modulating CXCL5/CXCR2 axis. Biochem Biophys Res Commun 2018;505:1032-7. [Crossref] [PubMed]
- Afrazi A, Branca MF, Sodhi CP, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem 2014;289:9584-99. [Crossref] [PubMed]
- Li P, Fu D, Sheng Q, et al. TUDCA attenuates intestinal injury and inhibits endoplasmic reticulum stress-mediated intestinal cell apoptosis in necrotizing enterocolitis. Int Immunopharmacol 2019;74:105665. [Crossref] [PubMed]
- Zhu X, Cui N, Yu L, et al. Potential role of endoplasmic reticulum stress is involved in the protection of fish oil on neonatal rats with necrotizing enterocolitis. Sci Rep 2020;10:6448. [Crossref] [PubMed]
- Li B, Lee C, Chuslip S, et al. Intestinal epithelial tight junctions and permeability can be rescued through the regulation of endoplasmic reticulum stress by amniotic fluid stem cells during necrotizing enterocolitis. FASEB J 2021;35:e21265. [Crossref] [PubMed]
- Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem 2010;285:38751-5. [Crossref] [PubMed]
- Weitkamp JH, Koyama T, Rock MT, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 2013;62:73-82. [Crossref] [PubMed]
- Ma F, Hao H, Gao X, et al. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics 2020;10:7730-46. [Crossref] [PubMed]
- Zhi L, Kim P, Thompson BD, et al. FTY720 blocks egress of T cells in part by abrogation of their adhesion on the lymph node sinus. J Immunol 2011;187:2244-51. [Crossref] [PubMed]
- Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol 2013;34:521-30. [Crossref] [PubMed]
- Lutz MB. Induction of CD4(+) Regulatory and Polarized Effector/helper T Cells by Dendritic Cells. Immune Netw 2016;16:13-25. [Crossref] [PubMed]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 2003;3:984-93. [Crossref] [PubMed]
- Hadis U, Wahl B, Schulz O, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011;34:237-46. [Crossref] [PubMed]
- Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830-5. [Crossref] [PubMed]
- Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 2006;441:231-4. [Crossref] [PubMed]
- Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008;453:236-40. [Crossref] [PubMed]
- Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967-74. [Crossref] [PubMed]
- Gaffen SL, Jain R, Garg AV, et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014;14:585-600. [Crossref] [PubMed]
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201:233-40. [Crossref] [PubMed]
- Pang Y, Du X, Xu X, et al. Monocyte activation and inflammation can exacerbate Treg/Th17 imbalance in infants with neonatal necrotizing enterocolitis. Int Immunopharmacol 2018;59:354-60. [Crossref] [PubMed]
- Zeng X, Wang T, Zhu C, et al. Topographical and biological evidence revealed FTY720-mediated anergy-polarization of mouse bone marrow-derived dendritic cells in vitro. PLoS One 2012;7:e34830. [Crossref] [PubMed]


