
Risk factors of cerebral palsy in children: a systematic review and meta-analysis
Introduction
Pediatric cerebral palsy is a clinical syndrome caused by brain injury or lesions in children from before birth to 1 month after birth, and mainly manifests as non-progressive central dyskinesia and abnormal posture (1,2). Research has shown that the prevalence of cerebral palsy in children worldwide is about 3.16% (3), and results in heavy familial and social burdens as well as usage of medical resources (4,5). Despite considerable research, the complex etiology and pathogenesis of cerebral palsy remain unclear. The high-risk factors of cerebral palsy are complex and diverse (6,7), and the time span of these risk factors is long, which can occur at birth as well as before or after birth. Moreover, these risk factors affect each other and form an intertwined network, leading to the occurrence of diseases (8,9). Unfortunately, previous research on risk factors for cerebral palsy in children has been limited and inconsistent. For example, a study was limited to at-birth and fetal factors (5). Perinatal factors and maternal factors are usually ignored (5). Whether gestational hypertension increases the risk of cerebral palsy in children has long been controversial. A study has pointed out that preterm birth leads to a significant increase in the incidence of cerebral palsy (7). One study has also pointed to neurodevelopmental impairment, rather than preterm birth, as a risk factor for cerebral palsy (6). Emergency cesarean section and premature rupture of membranes are risk factors for cerebral palsy in term infants, but the relationship between the three is not close in preterm infants. In addition, due to the development of maternal and neonatal medical and nursing techniques, the risk factors for pediatric cerebral palsy have changed, such as premature birth and the incidence of brain injury caused by hyperbilirubinemia has dropped sharply. In today’s medical context, identifying or updating risk factors for pediatric cerebral palsy can inform preventive interventions. Aiming at the above-mentioned controversy, this study conducted a meta-analysis of the literature published over the past 20 years exploring the pregnancy risk factors of mothers with infantile cerebral palsy, in order to find the main risk factors of infantile cerebral palsy. Our study calculated the odds ratio (OR) and 95% confidence interval (CI) to provide a scientific basis for the prevention and decision-making of infantile cerebral palsy. We present the following article in accordance with the MOOSE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-78/rc).
Methods
Bibliography retrieval
We performed literature searches of the PubMed, EMBASE, MEDLINE, and CENTRAL databases using the following search terms: (“cerebrl plsy” or “cerebrl plsis” or “infantile cerebral palsy”) and (“risk factors”).
Literature screening
The inclusion criteria were as follows: (I) case-control or cohort study; (II) the subjects of the study were children with cerebral palsy and healthy control children; (III) the subject of literature research is the risk factors of cerebral palsy in children; (IV) exposure factors include gestational hypertension, premature birth, premature rupture of membranes, or emergency cesarean section; (IV) there are risk ratio (RR) values or odds ratio (OR) values and corresponding 95% confidence intervals (CI) in the results of literature studies or can be calculated based on data..
The exclusion criteria were as follows: (I) studies with no control group; (II) literature studies other exposure factors; (III) the diagnostic criteria of the subjects were lacking or unclear; and (IV) case reports and replicate studies.
Document data sorting
In this study, a researcher sorted the basic information and data of the literature that met the inclusion criteria, which included the literature title, publication year, author information, statistical methods, published journals, OR value or RR value, 95% CI, etc. Another researcher conducted information verification and data proofreading to ensure the accuracy of the information and data.
Literature quality evaluation
In this paper, three researchers used the Newcastle-Ottawa scale (NOS) of included studies to evaluate the quality of the included studies in terms of the selection of subjects (4 points), intergroup comparability (2 points), and exposure factor measurement (3 points), with a total of 9 points. Three researchers jointly completed the literature quality evaluation. Disagreements were resolved by discussion and consensus.
Heterogeneity test
The Chi-square test was used to assess the heterogeneity of the included studies. When the corrected I2 value by degrees of freedom was >50% and P<0.1, it was considered that there was heterogeneity among the published literatures. Subgroup analysis was used to explore the causes of heterogeneity; if the cause of heterogeneity was not found, the random effects model was employed. However, when I2≤50% and P≥0.1 after degrees of freedom correction, it was considered that there was no heterogeneity among the published literature, and the fixed effects model was applied.
Analysis of sources of heterogeneity and testing for publication bias
If there is heterogeneity among the literatures, this study used subgroup analysis and sensitivity analysis to determine the source of heterogeneity. If subgroup analyses and sensitivity analyses could not identify the source of heterogeneity, no pooling between study results was performed, and only individual study results were described. In this study, Egger’s test was used to test for publication bias.
Statistical analysis
The Cochrane RevMan5.3 software (The Nordic Cochrane, Copenhagen) was used to analyze the data. The observation factors were statistically described using the OR value and 95% CI. Two-sided P<0.05 was considered to indicate statistical significance.
Results
Retrieval results and literature quality evaluation
In this study, 1,836 literatures related to the risk factors of cerebral palsy in children were retrieved from the databases. After screening according to the inclusion and exclusion criteria, 13 articles were included in the analysis, 3 cohort studies, 10 case control studies. There were a total of 2,489 children with cerebral palsy and 4,782 children without cerebral palsy. In terms of the NOS scores of the 13 included studies, none of the articles scored 9, four scored 8, eight scored 7, and one scored 6. The literature screening flow chart was shown in Figure 1 and basic information of the included studies was shown in Table 1.
Table 1
| Study | Study design | NOS |
|---|---|---|
| Drougia (4) 2007 | Case control | 8 |
| Bufteac (8) 2021 | Cohort | 8 |
| Gurbuz (9) 2006 | Case control | 7 |
| Ichizuka (10) 2021 | Case control | 7 |
| Kulak (11) 2010 | Case control | 7 |
| Livinec (3) 2005 | Cohort | 7 |
| Monokwane (12) 2017 | Case control | 8 |
| Moster (13) 2010 | Case control | 7 |
| Nielsen (14) 2008 | Case control | 6 |
| Stelmach (15) 2005 | Case control | 7 |
| Walstab (5) 2004 | Case control | 7 |
| Yuan (1) 2019 | Case control | 7 |
| Herbst (16) 2001 | Cohort | 8 |
Analysis of risk factors of cerebral palsy
Maternal pregnancy-induced hypertension
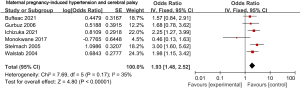
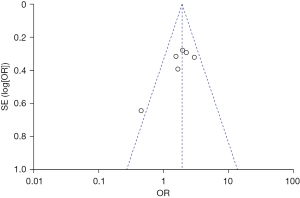
Six of the 12 included articles investigated the relationship between maternal pregnancy-induced hypertension and cerebral palsy. There was no heterogeneity among these six studies (χ2=7.69, P=0.17, I2=35%), and the fixed effects model was used. The combined OR value was 1.93, 95% CI was (1.48, 2.52), and the combined effect quantity was Z=4.80 (P<0.00001). Therefore, maternal gestational hypertension was identified as ana risk factor for cerebral palsy, as shown in Figure 2. Egger test showed no publication bias in the above six articles (P>0.05), as shown in Figure 3.
Premature rupture of membranes
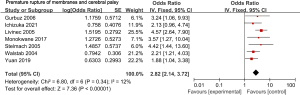
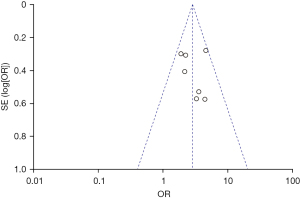
Seven of the 12 included articles studied the relationship between premature rupture of membranes and cerebral palsy. There was no heterogeneity among the 7 studies (χ2=6.80, P=0.34, I2=12%), and the fixed effects model was us ed. The combined OR value was 2.82, 95% CI was (2.14, 3.72), and the combined effect quantity was Z=7.36 (P<0.00001). Thus, premature rupture of membranes was identified as a risk factor for cerebral palsy in children, as shown in Figure 4. Egger test showed no publication bias in these seven articles (P>0.05), as shown in Figure 5.
Premature delivery
Seven of the 12 included articles examined the relationship between preterm birth and cerebral palsy. There was no heterogeneity among the seven studies (χ2=2.98, P=0.81, I2=0%), and the fixed effects model was used. The combined OR value was 2.90, 95% CI was (2.13, 3.93), and the combined effect quantity was Z=6.83 (P<0.00001). Hence, preterm birth was identified as a risk factor for cerebral palsy in children, as shown in Figure 6. Egger test showed no publication bias in these seven articles (P>0.05), as shown in Figure 7.
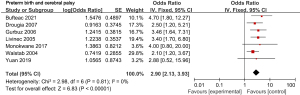
Emergency cesarean section
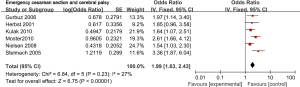
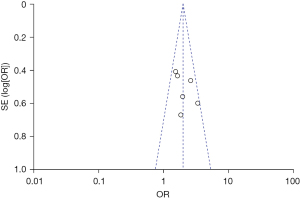
Six of the 12 included articles explored the relationship between emergency cesarean section and cerebral palsy. There was no heterogeneity among these six literatures (χ2=6.84, P=0.23, I2=27%), and the fixed effects model was used. The combined OR value was 1.99, 95% CI was (1.63, 2.43), and the combined effect amount was Z=6.75 (P<0.00001). Thus, emergency cesarean section was identified as a risk factor for cerebral palsy, as shown in Figure 8. Egger test showed no publication bias in these six articles (P>0.05), as shown in Figure 9.
Discussion
The pathogenic factors of cerebral palsy in children are complex. Comprehensive monitoring of various risk factors and timely intervention can significantly reduce the risk (17). Our study confirmed that maternal pregnancy-induced hypertension, premature rupture of membranes, premature delivery, and emergency cesarean section were risk factors for cerebral palsy in children.
Through meta-analysis, Himmelmann et al. (18) confirmed that maternal pregnancy-induced hypertension was a risk factor for cerebral palsy in children, which is consistent with our results. Research has pointed out that pregnancy-induced hypertension is primarily attributable a systemic arteriolar spasm, resulting in insufficient blood supply to the placenta and severe intrauterine hypoxia, fetal craniocerebral nerve injury, and even fetal neonatal death (19). In addition, a study has confirmed that the infants of pregnant mothers with pregnancy-induced hypertension have mental retardation, and the intelligence of these newborns is significantly lower than that of infants of normal pregnancy. In fact, there were still differences in intelligence between these two groups at adulthood (20). This study confirmed that gestational hypertension causes irreversible damage to the fetal nervous system. Timely symptomatic treatment and control of maternal blood pressure during pregnancy can effectively reduce the long-term disability risk of newborns (20).
A previous study has also shown a significant correlation between preterm birth and cerebral palsy in children (21). In recent 20 years, neonatal science has been continuously developing, resulting in increased survival rates among premature infants. The incidence rate of cerebral palsy has also increased (22,23). Epidemiological investigation has shown that preterm infants account for about 7% of all surviving newborns, but preterm cerebral palsy accounts for about 40% of cerebral palsy cases (21). Premature infants have immature development in all organs. Brain tissue is the most vulnerable to damage, and clinical manifestations are the most obvious (24,25). In addition, a study has indicated that the incidence rate of cerebral palsy is negatively correlated with gestational age; when the gestational week is less than 28 weeks, the risk of cerebral palsy increases about 70 times (17).
Through meta-analysis, O’Callaghan et al. (26) confirmed that emergency cesarean section was a risk factor of cerebral palsy, which is consistent with our results. The results of their study do not support that elective cesarean section, full-term cesarean section, premature cesarean section, and breech cesarean section were risk factors for cerebral palsy, although they are also not protective factors. Their study does not recommend emergency cesarean section to prevent cerebral palsy in children. Compared with emergency cesarean section, clinical monitoring should be strengthened and planned elective cesarean section should be selected. An additional study has pointed out that caesarean section can reduce the incidence and severity of ventricular hemorrhage in preterm infants (27). In the case of premature delivery, further research should be conducted.
Premature rupture of membranes is a risk factor for mother-infant infection and can eventually progress to preterm birth (28). Research has confirmed a correlation between the incidence of premature rupture of membranes and the incidence of cerebral palsy in children. In our research results, premature rupture of membranes was a risk factor for cerebral palsy, which is consistent with previous research findings. Our research clearly expounded on the relationship between premature rupture of membranes and cerebral palsy. Clinically, it is necessary to treat premature rupture of membranes according to a series of conditions, such as amniotic fluid pollution, gestational weeks, and fetal maturity. Appropriate treatment can reduce the incidence rate of cerebral palsy (29).
In addition to the risk factors of cerebral palsy in children determined in our study, other studies have identified low body weight, neonatal respiratory distress, maternal infection during pregnancy, neonatal infection, persistent jaundice, multiple births, and amniotic fluid pollution a risk factors of cerebral palsy (12,30). It should be noted that these factors do not exist independently but are intertwined. The emergence of a risk factor may result from the joint action of several other risk factors. For example, pregnancy-induced hypertension and premature rupture of membranes may cause preterm birth. Preterm infants can easily experience symptoms such as neonatal low weight and infection (31). This interactive role network dramatically increases the difficulty of disease monitoring and prevention.
This study identified the risk factors of cerebral palsy in children through meta-analysis, which provides a reference basis for risk monitoring and clinical intervention.
Acknowledgments
Funding: The project was supported by Hainan Province Clinical Medical Center.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-78/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-78/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yuan J, Wang J, Ma J, et al. Paediatric cerebral palsy prevalence and high-risk factors in Henan province, Central China. J Rehabil Med 2019;51:47-53. [Crossref] [PubMed]
- Toldi J, Escobar J, Brown A. Cerebral Palsy: Sport and Exercise Considerations. Curr Sports Med Rep 2021;20:19-25. [Crossref] [PubMed]
- Livinec F, Ancel PY, Marret S, et al. Prenatal risk factors for cerebral palsy in very preterm singletons and twins. Obstet Gynecol 2005;105:1341-7. [Crossref] [PubMed]
- Drougia A, Giapros V, Krallis N, et al. Incidence and risk factors for cerebral palsy in infants with perinatal problems: a 15-year review. Early Hum Dev 2007;83:541-7. [Crossref] [PubMed]
- Walstab JE, Bell RJ, Reddihough DS, et al. Factors identified during the neonatal period associated with risk of cerebral palsy. Aust N Z J Obstet Gynaecol 2004;44:342-6. [Crossref] [PubMed]
- Dan B. Cerebral palsy is a sensorimotor disorder. Dev Med Child Neurol 2020;62:768. [Crossref] [PubMed]
- Morgan P, McGinley JL. Cerebral palsy. Handb Clin Neurol 2018;159:323-36. [Crossref] [PubMed]
- Bufteac Gincota E, Jahnsen R, Spinei L, et al. Risk Factors for Cerebral Palsy in Moldova. Medicina (Kaunas) 2021;57:540. [Crossref] [PubMed]
- Gurbuz A, Karateke A, Yilmaz U, et al. The role of perinatal and intrapartum risk factors in the etiology of cerebral palsy in term deliveries in a Turkish population. J Matern Fetal Neonatal Med 2006;19:147-55. [Crossref] [PubMed]
- Ichizuka K, Toyokawa S, Ikenoue T, et al. Risk factors for cerebral palsy in neonates due to placental abruption. J Obstet Gynaecol Res 2021;47:159-66. [Crossref] [PubMed]
- Kułak W, Okurowska-Zawada B, Sienkiewicz D, et al. Risk factors for cerebral palsy in term birth infants. Adv Med Sci 2010;55:216-21. [Crossref] [PubMed]
- Monokwane B, Johnson A, Gambrah-Sampaney C, et al. Risk Factors for Cerebral Palsy in Children in Botswana. Pediatr Neurol 2017;77:73-7. [Crossref] [PubMed]
- Moster D, Wilcox AJ, Vollset SE, et al. Cerebral palsy among term and postterm births. JAMA 2010;304:976-82. [Crossref] [PubMed]
- Nielsen LF, Schendel D, Grove J, et al. Asphyxia-related risk factors and their timing in spastic cerebral palsy. BJOG 2008;115:1518-28. [Crossref] [PubMed]
- Stelmach T, Pisarev H, Talvik T. Ante- and perinatal factors for cerebral palsy: case-control study in Estonia. J Child Neurol 2005;20:654-60. [Crossref] [PubMed]
- Herbst A, Thorngren-Jerneck K. Mode of delivery in breech presentation at term: increased neonatal morbidity with vaginal delivery. Acta Obstet Gynecol Scand 2001;80:731-7. [Crossref] [PubMed]
- Zhao M, Dai H, Deng Y, et al. SGA as a Risk Factor for Cerebral Palsy in Moderate to Late Preterm Infants: a System Review and Meta-analysis. Sci Rep 2016;6:38853. [Crossref] [PubMed]
- Himmelmann K, Ahlin K, Jacobsson B, et al. Risk factors for cerebral palsy in children born at term. Acta Obstet Gynecol Scand 2011;90:1070-81. [Crossref] [PubMed]
- Blair E, Watson LAustralian Cerebral Palsy Register Group. Cerebral palsy and perinatal mortality after pregnancy-induced hypertension across the gestational age spectrum: observations of a reconstructed total population cohort. Dev Med Child Neurol 2016;58:76-81. [Crossref] [PubMed]
- Tanaka Y, Hayashi T, Kitajima H, et al. Inhaled nitric oxide therapy decreases the risk of cerebral palsy in preterm infants with persistent pulmonary hypertension of the newborn. Pediatrics 2007;119:1159-64. [Crossref] [PubMed]
- Bodensteiner JB, Johnsen SD. Magnetic resonance imaging (MRI) findings in children surviving extremely premature delivery and extremely low birthweight with cerebral palsy. J Child Neurol 2006;21:743-7. [Crossref] [PubMed]
- Kakooza-Mwesige A, Forssberg H, Eliasson AC, et al. Cerebral palsy in children in Kampala, Uganda: clinical subtypes, motor function and co-morbidities. BMC Res Notes 2015;8:166. [Crossref] [PubMed]
- Peixoto MV, Duque AM, Santos AD, et al. Spatial analysis of cerebral palsy in children and adolescents and its association with health vulnerability. Geospat Health 2020;15: [Crossref] [PubMed]
- Lammertink F, Vinkers CH, Tataranno ML, et al. Premature Birth and Developmental Programming: Mechanisms of Resilience and Vulnerability. Front Psychiatry 2020;11:531571. [Crossref] [PubMed]
- Gauda EB, McLemore GL. Premature birth, homeostatic plasticity and respiratory consequences of inflammation. Respir Physiol Neurobiol 2020;274:103337. [Crossref] [PubMed]
- O'Callaghan M, MacLennan A. Cesarean delivery and cerebral palsy: a systematic review and meta-analysis. Obstet Gynecol 2013;122:1169-75. [Crossref] [PubMed]
- Poryo M, Boeckh JC, Gortner L, et al. Ante-, peri- and postnatal factors associated with intraventricular hemorrhage in very premature infants. Early Hum Dev 2018;116:1-8. [Crossref] [PubMed]
- Gatta LA, Hughes BL. Premature Rupture of Membranes with Concurrent Viral Infection. Obstet Gynecol Clin North Am 2020;47:605-23. [Crossref] [PubMed]
- Drassinower D, Friedman AM, Običan SG, et al. Prolonged latency of preterm premature rupture of membranes and risk of cerebral palsy. J Matern Fetal Neonatal Med 2016;29:2748-52. [PubMed]
- Crisham Janik MD, Newman TB, Cheng YW, et al. Maternal diagnosis of obesity and risk of cerebral palsy in the child. J Pediatr 2013;163:1307-12. [Crossref] [PubMed]
- Bear JJ, Wu YW. Maternal Infections During Pregnancy and Cerebral Palsy in the Child. Pediatr Neurol 2016;57:74-9. [Crossref] [PubMed]




