
Permanent epicardial pacing in neonates and infants less than 1 year old: 12-year experience at a single center
Introduction
Advancing surgical techniques applied to complex cardiac anatomy have allowed palliative and reparative interventions in very small infants, thus increasing the population of infants requiring permanent pacing (1). Due to their young age, low weight, abnormal cardiac structure and vascular access, it is difficult to implant endocardial pacing leads, and the implantation of epicardial pacing leads becomes an ideal method (2,3). Despite major advances in pacemaker lead and generator technology (4,5), outcomes following implantation of epicardial permanent pacemakers (EPPMs) in neonates and small infants seem to be less satisfactory than in slightly older patients (6). However, little has been published on modern pacemaker therapy in this age group. The objective of the present study was to report our mid-term experience in neonates and infants and to evaluate the safety of epicardial pacing. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-525/rc).
Methods
Patients
We included consecutive patients who underwent EPPM implantation from Dec 1, 2008 through Dec 1, 2019 at Guangdong Provincial People’s Hospital for this observational cohort study. Patients aged >1 year old at the time of EPPM implantation were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital [No. GDREC2019338H(R2)] and individual consent for this retrospective analysis was waived. We retrospectively reviewed data that had been prospectively collected, including demographic data, hospital records, surgical implantation/revision records and follow-up files.
Operative course
We used unipolar steroid-eluting epicardial pacing leads (CapSure Epi-4965; Medtronic, Minneapolis, Minnesota) and generators with automatic threshold measurement (AutoCapture; St. Jude Medical, Sylmar, Los Angeles, California) for all patients. The steroid-eluting leads can lower the pacing threshold by reducing the inflammation and fibrosis (7). The generator mode was ventricular (VVI/R) in 29 (93.5%) and dual-chamber (DDD/R) in 2 (6.5%) patients. The surgical approach for the pacing leads was left lateral thoracotomy (26/31 patients, 83.9%), median sternotomy (3/31, 9.7%) or right lateral thoracotomy (2/31, 6.5%). Left lateral thoracotomy was preferred, but median sternotomy was used in pacemaker implantation performed at the time of cardiac surgery. It is widely accepted that median sternotomies lead to greater damage to the epicardial wall, resulting in relatively more fibrosis, dense scarring, adhesions, and inflammation (8). We made an effort to find a suitable area for lead implantation, with more extensive tissue dissection around the epicardium and lateral thoracotomy to approach areas not accessed in previous surgeries. Usually, a left lateral thoracotomy can directly reach the surgical site, obtain excellent exposure, shorten operation time, and get small trauma, which was still the first choice when managing the reoperations. We implanted most ventricular leads (30/31, 96.8%) in the anterior wall of the left ventricle (LV) to avoid right ventricle (RV) pacing-induced ventricular dysfunction (9,10). We implanted 2 (4.5%) atrial leads on the right atrium (Table 1), and created a generator pocket above the rectus sheath via a left subcostal (29/31, 93.5%) or median abdominal (2/31, 6.5%) incision. A segment of pacing wire was reserved in the chest cavity to prevent lead fracture and displacement caused by growth of neonates and infants. Before closing the pocket, we tested the pacing threshold to confirm that the pacemaker was functioning properly.
Table 1
| Variables | Overall (n=31) |
|---|---|
| Male/female | 18/13 |
| Age at operation, years | 0.4 (0.4) |
| Weight, kg | 5.3 (3.5) |
| Height, cm | 61.0 (15.0) |
| Premature | 5 (16.1%) |
| Cesarean delivery | 12 (38.7%) |
| Cardiac anatomy | |
| VSD | 12 (38.7%) |
| DORV | 6 (19.4%) |
| ASD | 3 (9.7%) |
| TGA | 2 (6.5%) |
| TOF | 1 (3.2%) |
| TA | 1 (3.2%) |
| Indication for pacing | |
| Postoperative AVB | 21 (67.7%) |
| Congenital AVB | 9 (29.0%) |
| Myocarditis | 1 (3.2%) |
| Combined cardiac surgery | 1 (3.2%) |
| Surgical approach | |
| Left sternotomy | 26 (83.9%) |
| Right sternotomy | 2 (6.5%) |
| Median sternotomy | 3 (9.8%) |
| Lead position | |
| Left ventricle | 28 (90.3%) |
| Left ventricle and atrium | 2 (6.5%) |
| Right ventricle | 1 (3.2%) |
| Pacing mode | |
| VVI/R | 29 (93.5%) |
| DDD/R | 2 (6.5%) |
| Hospital stay after operation, days | 12.0 (8.0) |
| ICU stay, days | 1.0 (1.0) |
| Ventilator, hours | 7.0 (33.0) |
| In-hospital mortality | 1 (3.2%) |
Continuous variables are expressed as median (IQR), categorical variables as percentage. VSD, ventricular septal defect; DORV, double outlet of right ventricle; ASD, atrial septal defect; TGA, transposition of great arteries; TOF, tetralogy of Fallot; TA, tricuspid atresia; AVB, atrioventricular block; VVI, ventricular demand mode; DDD, atrioventricular synchronized pacing; R, rate modulation; ICU, intensive care unit.
Follow-up
There was follow-up of all patients who were discharged alive, and a questionnaire was used to collect baseline clinical and demographic data from each patient. Pacing threshold and lead impedance were obtained through follow-up of pacemaker programming control within 3 months after implantation and at 6-month intervals thereafter. Echocardiographic measurements for left ventricular size and function were obtained at each follow-up. For patients using other centers for follow-up, we contacted their parents or guardians to obtain their health status, cardiac function and pacemaker reoperation. There were no missing follow-up patients in this study. The local registry offices were contacted to ascertain mortality and acquire death certificates.
All-cause mortality was defined as death due to any cause during follow-up. Lead failure was defined as a need for replacement based on increasing pacing threshold or lead impedance. Premature battery depletion was indicated when generator failure at factory settings occurred at less than its minimum projected longevity minus 2 years.
Statistical analysis
Continuous variables were tested for normality using the Shapiro-Wilk test and were displayed as medians [interquartile range (IQR)] or means [standard deviation (SD)] as appropriate according to the data distribution. Independent t-tests were performed for normally distributed variables, and Mann-Whitney U tests otherwise. Paired variables conforming to normality were compared using paired Student’s t-tests. The Wilcoxon signed rank test or the marginal homogeneity test was used for paired variables failing to conform to normality. Survival and freedom from generator and lead reoperation after first implantation were analyzed with the Kaplan-Meier method. R software (version 4.1.0) was used for data analysis. A two-tailed P<0.05 was considered to be statistically significant.
Results
Patients
Patients underwent EPPM implantation within the first year of life between 1 December 2008 and 1 December 2019. Out of a total of 31 consecutive patients, 2 (6.5%) were neonates and 18 (58.1%) were boys. The median age and weight of the patients at the time of operation were 156 days (IQR 217) and 5.3 kg (IQR 3.5), respectively. 25 (80.6%) patients had congenital heart malformation, 17 (54.8%) of whom had complex heart disease. Structural diagnoses of the patients are summarized in Table 1. Indications for permanent pacemaker implantation were postoperative atrioventricular block (AVB) in 21 (67.7%) of the patients, congenital AVB in 9 (29.0%), and myocarditis-induced AVB in 1 (3.2%). Patients were followed up for a median of 3.9 years (IQR 4.7) after the first EPPM implantation. None of patients were lost to follow-up.
Mortality
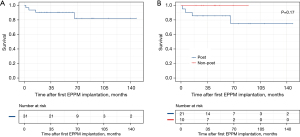
There was one early in-hospital death and three late deaths in the study population. The patient survival curve is shown in Figure 1A. All had undergone correction of intracardiac malformations before receiving pacemakers. The cause of the early death was pulmonary infection with an original diagnosis of DORV (double outlet right ventricle). Among the late deaths, one patient with VSD (ventricular septal defect) died of pulmonary infection within a year of discharge, and one with TOF (tetralogy of Fallot) died of malignant arrhythmia in the second year after surgery. The remaining patient never visited the hospital after having been discharged with a diagnosis of DORV with PS (pulmonary stenosis). To determine the effect of cardiac surgery on patient prognosis, we divided patients into two groups, one with postoperative AVB (n=21) and the other with non-postoperative AVB (n=10). Previous cardiac surgery did not significantly affect survival time (P=0.17, Figure 1B). Likewise, we divided patients into three groups according to cardiac anatomy: normal structure (n=6), simple congenital heart disease [isolated VSD or atrial septal defect (ASD)] (n=8) and complex congenital heart disease (n=17). Survival time showed no significant difference in the three groups (P=0.55).
Perioperative course
The postoperative hospital stay, intensive care unit (ICU) stay and mechanical ventilation time were 12 days (IQR 8), 1 day (IQR 1) and 7 hours (IQR 33), respectively (Table 1). No significant differences were observed between patients with postoperative AVB and non-postoperative AVB in postoperative hospital stay (P=0.899), ICU stay (P=0.578) or time on mechanical ventilation (P=0.671). We also compared the above three indicators of cardiac anatomy and did not find a significant difference between the three groups (P=0.811, P=0.447, P=0.457, respectively).
Early complications in patients included pulmonary infection in 6 (19.4%), pleural effusion in 3 (9.7%), abdominal distention in 2 (6.5%), pocket infection in 1 (3.2%) and catheter-related infection in 1 (3.2%). Pocket infection occurred in an 8-day-old neonate with congenital AVB. We changed the generator, lead, and pocket for this patient, who then underwent small bowel resection due to septic shock leading to necrotizing enteritis, and we eventually changed to total transvenous pacing.
Follow-up of EPPM implantation
During follow-up, pacing generators were replaced without a mode change 17 times in 12 (38.7%) patients (Table 2). Generator migration occurred in a 3-day-old neonate in the third year after surgery (Figure 2). The median longevity of a generator was 3.3 years (IQR 2.8). Freedom from generator replacement after first implantation was 90.3%, 75.6%, 52.4% and 43.6% at 1, 3, 5 and 6 years, respectively (Figure 3A). Reasons for generator change included battery depletion (n=15), pocket infection (n=1) and generator migration (n=1).
Table 2
| Variables | Overall (n=31) |
|---|---|
| Follow-up time, years | 3.9 (4.7) |
| Reoperation | |
| Lead | 8 (25.8%) |
| Generator | 12 (38.7%) |
| At least one reoperation | 13 (41.9%) |
| Discharge | |
| Pacing threshold, v | 1.00 (0.25) |
| Lead impedance, Ω | 358 [108] |
| Lower rate, beats | 110 [20] |
| Last follow-up | |
| Pacing threshold, v | 1.00 (0.60) |
| Lead impedance, Ω | 333 [100] |
| Lower rate, beats | 90 [20] |
Continuous variables are expressed as median (IQR), categorical variables as percentage. IQR, interquartile range.
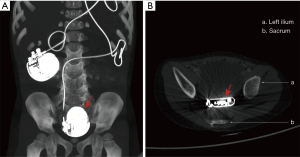
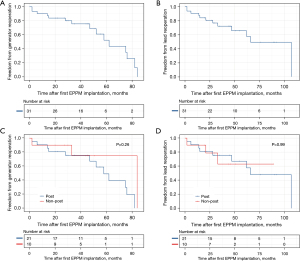
Pacing leads were changed 11 times in 8 (25.8%) patients without changing the type of lead. Median longevity of leads was 2.9 years (IQR 3.6). Freedom from lead replacement after the first implantation was 90.3%, 72.0%, 65.5% and 49.1% at 1, 3, 5, and 8 years, respectively (Figure 3B). There were 9 lead failures in 7 (22.6%) patients, resulting from lead fracture (n=1), lead adhesion (n=1), lead displacement (n=1), poor electrical contacts (n=2) or other causes leading to major increases in pacing threshold or impedance (n=4). One patient developed an increase in pacing threshold after 2 years because of spontaneous recovery from complete AVB, and as a result the pacing system was turned off. Otherwise, there were no significant differences in pacing threshold (P=0.482) and lead impedance (P=0.221) at the final follow-up compared with those at discharge. In order to identify the effect of different surgical approaches, we compared the prognosis among different surgical approaches, and no statistical difference was found in generator (P=0.759) and lead (P=0.956) reoperation.
Freedom from generator reoperation in patients with postoperative AVB was 90.5% and 75.6% at 1 and 3 years, respectively, and for patients with non-postoperative AVB was 90.0% and 75.0%, respectively. The results for the two groups did not differ significantly (Figure 3C, P=0.26). In terms of leads, freedom from lead reoperation in patients with postoperative AVB was 90.5%, 81.0%, and 75.6% at 1, 2, and 3 years, respectively, and for patients with non-postoperative AVB was 90.0%, 78.7%, and 63.0%, respectively. The results for the two groups did not differ significantly (Figure 3D, P=0.99). In patients grouped by cardiac anatomy, we also did not find significant differences between the three groups in generator (P=0.38) or lead (P=0.40) reoperation.
Follow-up echocardiography
Left ventricular dimensions were normal at implantation in 21 (67.8%) patients. Mild dilatation of the left ventricle was observed in 5 (16.1%) patients, and severe dilatation was observed in 5 (16.1%) patients. There were 6 patients whose left ventricular dilation was relieved or they recovered over time. One patient with normal left ventricular dimensions before surgery developed mild left ventricular dilation after 4 years. The left ventricular end-diastolic diameter (LVEDD) at the final follow-up was 32.0 mm (IQR 13.4), which was significantly greater than the preoperative LVEDD [23.0 mm (IQR 6.0), P<0.001] (Table 3). Similarly, left ventricular end systolic diameter (LVESD) was significantly greater at the final follow-up versus the preoperative value [19.0 mm (IQR 7.0) vs. 13.8 mm (IQR 5.0), respectively, P<0.001]. Left ventricular function decreased in 1 patient with a left ventricular ejection fraction (LVEF) of 45% after 2 years. There was no statistically significant difference between preoperative LVEF and that at the final follow-up [69.0% (IQR 14.0) vs. 71.0% (IQR 11.0), respectively, P=0.657]. However, tricuspid regurgitation (TR) at the final follow-up was significantly less than before the operation [0 (IQR 1) vs. 1 (IQR 2), respectively, P=0.039]. We did not observe significant effects of previous cardiac surgery on LVEDD (P=0.849), LVESD (P=0.553) or LVEF (P=0.626). Congenital heart disease also did not significantly affect the above three parameters (P=0.718, P=0.902, P=0.868, respectively).
Table 3
| Variables | Preoperative | Final follow-up | P value |
|---|---|---|---|
| LVEF (%) | 69.0 (14.0) | 71.0 (11.0) | 0.657 |
| LVEDD, mm | 23.0 (6.0) | 32.0 (13.4) | <0.001 |
| LVESD, mm | 13.8 (5.0) | 19.0 (7.0) | <0.001 |
| TR | 1 [2] | 0 [1] | 0.039 |
| MR | 0 [1] | 0 [0] | 0.564 |
Continuous variables are expressed as median (IQR). TR and MR are divided into 6 grades according to severity, which are represented by 0–6 from mild to severe. P values indicate comparisons between preoperative and final follow-up echocardiography using Wilcoxon signed rank test or Marginal Homogeneity test as appropriate. LVEF, left ventricular ejection fraction; LVEDD, left ventricular end systolic diameter; LVESD, left ventricular end systolic diameter; TR, tricuspid regurgitation; MR, mitral regurgitation; IQR, interquartile range.
Discussion
Few studies have focused on prognosis and mid-term outcomes in neonates and infants with implanted EPPMs. In our observational study of patients who were followed up for as long as 12 years, we found that neonates and infants with EPPMs are at risk of repeated reoperations and all-cause death. Regular follow-up, type of pacing lead and congenital heart malformations, especially complicated congenital heart disease, may be closely related to a patient’s prognosis.
There was one in-hospital death and three late deaths in our study population. All had undergone surgical corrections of cardiac abnormalities, including one case of simple and three of complex congenital heart disease. These results suggest that congenital heart malformations, especially complicated congenital heart disease, may have a significant effect on prognosis in neonates and infants with implanted EPPMs. Although no obvious differences were observed, this may have resulted from the limited sample size, which could be addressed by a multi-center large-sample prospective study. In addition, all the late deaths had not been followed up regularly and were from low-income families with little education, thus reducing access to high-quality medical resources. For these young patients who have to undergo lifelong pacing, steps should be taken to reduce economic barriers, make them aware of risk factors for adverse events, and train them in cardiopulmonary resuscitation. We also observed an increase in LVEDD and LVESD, which may be related to growth and development of the young patients. The decrease in TR and normal LVEF in the follow-up period suggests that early EPPM implantation may have a beneficial effect on preservation of cardiac function.
Compared with the study of permanent epicardial pacing in older children (11), the lead longevity was shorter in our patients [10.8 years (SD 0.8) vs. 2.9 years (IQR 3.6), respectively]. Previous studies showed that the risk factors for primary lead dysfunction were young age (12,13) and structural heart disease (14,15). On the one hand, the frequent lead failures in neonates and infants can be explained by rapid growth in early childhood and increased physical activity resulting in an increase in mechanical stress on the epicardial leads. The mean age of the older children in the study referred to above was 5.7 years (SD 4.8), which is older than the age of our patients [0.4 years (IQR 0.6)]. On the other hand, most of our patients had congenital heart malformations and may have undergone more than one cardiac surgery. Epicardial fibrosis, scar, and myocardial degeneration after cardiac surgery can induce exit block and a high pacing threshold (16).
In addition, we found that lead longevity in a study using unipolar steroid-eluting leads for patients under 1 year old was 2.9 years (SD 1.9) (11), which is the same as that in our patients [2.9 years (IQR 3.6)]. But in another study using bipolar steroid-eluting leads for 22 neonates and infants, only one ventricular lead had to be replaced after 3.25 years during a median follow-up time of 4.6 years (17). Compared to that result, lead longevity was far shorter in our center. Furthermore, a recent study reported outcomes of 119 patients who underwent EPPM implantation at <18 years of age, in which intervention-free survival of the bipolar Medtronic 4968 lead was longer than that of unipolar leads from the same company (survival probability 94.0 vs. 58.3% at 8 years, respectively, P<0.001) (18). Intervention-free survival of the unipolar leads was similar to that of our study (49.1%). This result may be attributed to the superior design of the bipolar lead, which can leave more protected different lead wire available for unipolar pacing while damage occurred predominantly in the indifferent lead wire. Epicardial leads used in domestic centers are unipolar because bipolar leads have not been approved by National Medical Products Administration in China. It is recommended that the type of lead be optimized for patients in this age group.
Compared to older children with implanted unipolar steroid-eluting leads (11), generator longevity for our patients was shorter [3.3 (IQR 2.8) years vs. 5.5 (SD 0.3) years, respectively], although longevity was close to that in the patients under 1 year old in the above study [3.3 (IQR 2.8) years vs. 2.9 (SD 1.3) years, respectively]. In another study on both unipolar and bipolar steroid-eluting leads in older children, it was shown that younger age at the time of implantation may be one of the factors decreasing battery life (18). One important reason may be that EPPMs in younger patients need to generate a more rapid heart rate than those in older patients (19). Furthermore, high pacing threshold or lead impedance resulting from lead failure can shorten battery life. Interestingly, there seems to be a gender difference in generator reoperation [boys vs. girls: 13/18 (72.2%) vs. 4/13 (30.8%), respectively]. A similar phenomenon was observed in another study of older children with EPPM implantation, which demonstrated that male gender was a risk factor for having to transition earlier to a transvenous system (18). We suspect that this increased risk may result from more sports activities in boys, requiring higher pacing rates. Limited by the sample size, we cannot include more factors for multivariate analysis.
There were 2 cases of DDD/R mode in the current study. Although the dual chamber more closely resembles normal cardiac physiology (20), it has been reported that dual chamber mode does not influence battery longevity in comparison to single chamber (VVI/R) pacing (18). When choosing pacing mode, some authors have suggested that VVI/R pacing is more suitable for children with isolated complete heart block (19). DDD/R is an attractive alternative for patients who have congenital heart disease and suffer from iatrogenic AVB after surgical repair (21). We did not compare the two modes due to the limited sample size.
The epicardial pulse generator usually was placed above the rectus sheath. Neonates and premature babies of low body weight frequently have very thin rectus muscles. We experienced one generator migration into the pelvic cavity in a neonate, which were discovered 3 years after implantation. One option to prevent this movement would be to place the generator in the abdominal cavity (17). Given the risk of gut obstruction and erosion, it seems prudent to secure the generator to the internal abdominal wall with a suture. Another approach would be to place the generator in the retrosternal space (6). However, this method necessitates further follow-up for these patients since the pulse generator may compress mediastinal structures.
This study had several limitations. First, a multi-center and large-sample prospective study will be required to further divide the complex congenital heart disease in detail and identify risk factors for poor prognosis. Second, all leads were unipolar steroid-eluting leads. We recommend that the relevant government departments approve bipolar steroid-eluting epicardial pacing leads. And finally, limited by the sample size, it is difficult to include more factors for multivariate analysis. More neonates and infants with implanted EPPMs should be enrolled in subsequent studies.
Conclusions
The mid-term outcomes of EPPM implantation in neonates and infants were acceptable. However, neonates and infants with implanted EPPMs still have to face the risk of repeated reoperations and all-cause death. Regular follow-up, type of pacing lead and the presence of congenital heart malformations, especially complex congenital heart disease, may be closely related to patient prognosis.
Acknowledgments
We would like to thank the doctors, nurses, and social workers who contributed to this study.
Funding: This work was supported by National Natural Science Foundation of China (No. 82000081), National Key Research and Development Program of China (No. 2018YFC1002600), and Scientific Research Project of Guangdong Bureau of Traditional Chinese Medicine (No. 20211004). The funders had no influence in any part of the study and the researchers were independent from the funders.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-525/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-525/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-525/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sliz NB Jr, Johns JA. Cardiac pacing in infants and children. Cardiol Rev 2000;8:223-39. [Crossref] [PubMed]
- Beaufort-Krol GC, Mulder H, Nagelkerke D, et al. Comparison of longevity, pacing, and sensing characteristics of steroid-eluting epicardial versus conventional endocardial pacing leads in children. J Thorac Cardiovasc Surg 1999;117:523-8. [Crossref] [PubMed]
- Cohen MI, Bush DM, Vetter VL, et al. Permanent epicardial pacing in pediatric patients: seventeen years of experience and 1200 outpatient visits. Circulation 2001;103:2585-90. [Crossref] [PubMed]
- Bauersfeld U, Nowak B, Molinari L, et al. Low-energy epicardial pacing in children: the benefit of autocapture. Ann Thorac Surg 1999;68:1380-3. [Crossref] [PubMed]
- Tomaske M, Gerritse B, Kretzers L, et al. A 12-year experience of bipolar steroid-eluting epicardial pacing leads in children. Ann Thorac Surg 2008;85:1704-11. [Crossref] [PubMed]
- Kwak JG, Cho S, Kim WH. Surgical Outcomes of Permanent Epicardial Pacing in Neonates and Young Infants Less Than 1 Year of Age. Heart Lung Circ 2019;28:1127-33. [Crossref] [PubMed]
- Mond HG, Helland JR, Stokes K, et al. The electrode-tissue interface: the revolutionary role of steroid-elution. Pacing Clin Electrophysiol 2014;37:1232-49. [Crossref] [PubMed]
- Noiseux N, Khairy P, Fournier A, et al. Thirty years of experience with epicardial pacing in children. Cardiol Young 2004;14:512-9. [Crossref] [PubMed]
- Matsuhisa H, Oshima Y, Maruo A, et al. Pacing therapy in children. Circ J 2014;78:2972-8. [Crossref] [PubMed]
- Thambo JB, Bordachar P, Garrigue S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 2004;110:3766-72. [Crossref] [PubMed]
- Kwak JG, Kim SJ, Song JY, et al. Permanent epicardial pacing in pediatric patients: 12-year experience at a single center. Ann Thorac Surg 2012;93:634-9. [Crossref] [PubMed]
- Post MC, Budts W, Van de Bruaene A, et al. Failure of epicardial pacing leads in congenital heart disease: not uncommon and difficult to predict. Neth Heart J 2011;19:331-5. [Crossref] [PubMed]
- Paech C, Kostelka M, Dähnert I, et al. Performance of steroid eluting bipolar epicardial leads in pediatric and congenital heart disease patients: 15 years of single center experience. J Cardiothorac Surg 2014;9:84. [Crossref] [PubMed]
- Fortescue EB, Berul CI, Cecchin F, et al. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm 2004;1:150-9. [Crossref] [PubMed]
- Murayama H, Maeda M, Sakurai H, et al. Predictors affecting durability of epicardial pacemaker leads in pediatric patients. J Thorac Cardiovasc Surg 2008;135:361-6. [Crossref] [PubMed]
- Karpawich PP, Walters H, Hakimi M. Chronic performance of a transvenous steroid pacing lead used as an epi-intramyocardial electrode. Pacing Clin Electrophysiol 1998;21:1486-8. [Crossref] [PubMed]
- Aellig NC, Balmer C, Dodge-Khatami A, et al. Long-term follow-up after pacemaker implantation in neonates and infants. Ann Thorac Surg 2007;83:1420-3. [Crossref] [PubMed]
- Kubus P, Materna O, Gebauer RA, et al. Permanent epicardial pacing in children: long-term results and factors modifying outcome. Europace 2012;14:509-14. [Crossref] [PubMed]
- McLeod KA. Cardiac pacing in infants and children. Heart 2010;96:1502-8. [Crossref] [PubMed]
- Dretzke J, Toff WD, Lip GY, et al. Dual chamber versus single chamber ventricular pacemakers for sick sinus syndrome and atrioventricular block. Cochrane Database Syst Rev 2004;CD003710. [PubMed]
- Zhang T, Liu Y, Zou C, et al. Single chamber permanent epicardial pacing for children with congenital heart disease after surgical repair. J Cardiothorac Surg 2016;11:61. [Crossref] [PubMed]


