
Trends and risk factors of extended-spectrum beta-lactamase urinary tract infection in Chinese children: a nomogram is built and urologist should act in time
Introduction
Urinary tract infection (UTI) is the most common infection among young infants and children. They can be associated with long-term complications such as renal scarring and chronic renal failure whereby early treatment is needed (1).
UTIs are most frequently due to Enterobacteriaceae, mainly Escherichia coli (2). However, Escherichia coli species have variable antimicrobial resistance mechanisms which may include the production of extended-spectrum-β-lactamase (ESBL). ESBL are plasmid mediated enzymes that degrade penicillins, cephalosporins but spare cephamycins (cefoxitin, cefotetan), moxalactam and carbapenems and are mostly produced by Enterobacteriaceae (3). A recent systematic review and meta-analysis has shown a 14% pooled prevalence of pediatric UTI caused by ESBL producing Enterobacteriaceae in different countries (4). The emergence of ESBL-producing Enterobacteriaceae in pediatric urinary tract infections presents a serious threat to public health because no oral antibiotic as first-line treatment is regularly active against ESBL UTI and there are few intravenous options.
Thus, knowledge of the microorganisms involved in UTI and risk factors of urinary tract infections caused by ESBL-producing bacteria in children is important (5). Several limited studies have described the risk factors of ESBL UTI in children (6-8). Among the risk factors were underlying disease, patients with vesicoureteric reflux, UTI prophylaxis, previous UTI, hospitalization within the last three months, recent antibiotic use and high UTI recurrence rate.
A systematic review and meta-analysis reported that UTI is more prevalent in malnourished children than in their well-nourished counterparts (9). Another study included 402 malnourished children showed that more than 37% of UTI isolates were exhibiting extended spectrum beta lactamase (ESBL) phenotype (10). A retrospective study reviewed microbial etiologies and antimicrobial resistance among patients experiencing UTI events in the neurology ward and ESBL-producing K. pneumoniae increased significantly (11). To our knowledge, until now, whether urodynamic abnormality, undernourishment and underlying neurologic disorders were independent risk factors for ESBL positive UTI is still largely unknown. Furthermore, using multivariable logistic regression coefficients to construct a corresponding nomogram and predict the probability of ESBL(+) UTI is also needed.
Recently, we noticed a substantial increase in the incidence of ESBL UTI in our children at our institution. This prompted us to investigate the risk factors of acquisition of ESBL UTI and study their clinical characteristics, antimicrobial resistance in comparison to children with non- ESBL UTI. Determination of these risk factors will help us in choosing the appropriate initial antibiotic, preventing sub-optimal treatment and antimicrobial resistance. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-21-523/rc).
Methods
This is a retrospective case control study conducted at Chongqing Medical University Affiliated Children’s Hospital. Clinical data of patients with UTI were reviewed via an electronic medical records system for patients who were admitted to the urology ward of the Chongqing Medical University Affiliated Children’s Hospital in Chongqing, China between January 1994 and December 2019.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Board of Children’s Hospital of Chongqing Medical University (No. 22020278) and individual consent for this retrospective analysis was waived.
The age, gender, fever, side of the lesion, underlying renal anomalies, vesicoureteral reflux, urodynamic change, underlaying neurologic disorders, previous UTI in the last three months, previous antibiotics administration in the last three months, nutritional status, microbial cultures, antibiotic sensitivity of a urinary specimen and main diagnosis during admission to the urology ward were obtained.
When more than one urological malformation was identified in one patient, the most severe one was used for analysis. Depending on the location of the congenital urological malformations, we categorized side of the lesion into right, left, bilateral or unknown.
Urine specimens were obtained according to the American Academy of Pediatrics (AAP) guidelines by bladder catheterization in young children and by mid-stream sampling in toilet trained children (12). One urine culture was used for each patient. Mixed growths of cultures were excluded.
Positive urine culture was more than 50,000 colony-forming units/mL. UTI was defined as abnormal urine analysis and a positive urine culture according to the American Academy of Pediatrics guidelines. Identification and susceptibility profiling of all bacteria from positive urine cultures were determined by using an automated susceptibility system VITEK 2 (Bio Merieux, Marcy l Etoile, France) according to the Clinical and Laboratory Standard Institute guidelines. Asymptomatic bacteriuria or no identified bacterium in a urinary culture was excluded from the study. Malnutrition was defined as below −2 SD score from the median weight-for-age of the reference population.
Statistical analysis
A descriptive statistical analysis was performed. Chi-squared test was used to evaluate qualitative variables, and unpaired t-test was used for quantitative data evaluation. Logistic regression was used to determine risk factors for ESBL-producing bacteria UTI over the 26-year study period. A predictive model (nomogram) was also built using statistical software R (The R Foundation for Statistical Computing, Vienna, Austria). SPSS 19 was used to analyze the collected data. P values <0.05 was considered significant in all statistical analyses.
Results
Clinical characteristics of urological patients with UTI
A total of 854 children with UTI over 26 years were identified included 614 (71.90%) patients with congenital urological malformations (hydronephrosis: n=305, vesicoureteral reflux: n=136, neurogenic bladder: n=44, duplex kidney: n=41, bladder diverticulum: n=9, posterior urethral valves: n=17, intravesical ureterocele: n=34, prostatic utricle: n=7, urethral diverticulum: n=3, phimosis: n=2, renal hypoplasia: n=8, congenital megaureter: n=2, exstrophy of bladder: n=6) and 240 patients with no underlying disease. Of these, 457 (53.5%) patients had a first episode of UTI and 397 (46.5%) had recurrent UTI. The median age at presentation was 1.5 years (IQR =5.0). Males were predominant (64.4%).
The prevalence of ESBL (+) pathogens in UTI
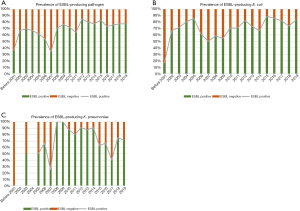
In our study of hospitalized patients with UTI over a 26 years interval 72.64% (377/519) were ESBL producers which is a high percentage. We analyzed whether the prevalence of antibiotics-resistant pathogens in UTI had increased over the past 26 years. Interestingly, the presence of ESBL-producing UTI increased significantly over the past 26 years (P=0.003). Among the isolated bacteria, the presence of ESBL-producing pathogens in E. coli increased significantly (P=0.021). However, ESBL in K. pneumoniae did not change over the last 26 years (P=0.482) (Figure 1).
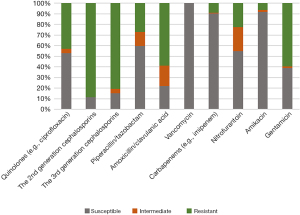
The 26-year overall susceptibility of UTI cases to individual antibiotics was as follows: 52.90% to quinolones (levofloxacin and ciprofloxacin), 11.22% to the 2nd generation cephalosporins, 15.18% to the 3rd generation cephalosporins, 59.94% to piperacillin/tazobactam, 21.86% to amoxicillin/clavulanic acid, 100% to vancomycin, 90.10% to carbapenems (e.g., imipenem, meropenem and ertapenem), 54.76% to nitrofurantoin, 91.90% to amikacin and 38.95% to gentamicin (Figure 2).
Risk factors for acquisition of ESBL producing UTI
Regarding the risk factors, the following were statistically significantly associated with ESBL-UTI: presence of congenital urological abnormalities (P=0.002), presence of vesicoureteral reflux (P=0.041), presence of neurologic disorder (P=0.011), age <12 months (P=0.002), fever (P=0.006) and previous use of antibiotics in the last 3 months (P=0.000) (Table 1).
Table 1
| Risk factors | ESBL (−), n (%) | ESBL (+), n (%) | χ2 | P value |
|---|---|---|---|---|
| Total (n=519) | 142 | 377 | ||
| Patients with congenital urological malformations (n=381) | 89 | 292 | 9.79 | 0.002* |
| Patients with no urological malformations (n=138) | 52 | 86 | ||
| Patients with vesicoureteral reflux (n=102) | 19 | 83 | 4.158 | 0.041* |
| Patients with no vesicoureteral reflux (n=417) | 122 | 295 | ||
| Patients with urodynamic change (n=38) | 10 | 28 | 0.000 | 1.000 |
| Patients with no urodynamic change (n=481) | 131 | 350 | ||
| Patients with neurologic disorder (n=32) | 2 | 30 | 6.457 | 0.011* |
| Patients with no neurologic disorder (n=487) | 139 | 348 | ||
| Patients with undernourishment | 2 | 12 | 1.969 | 0.425 |
| Patients with no undernourishment | 140 | 365 | ||
| Age <12 months (n=241) | 49 | 192 | 9.99 | 0.002* |
| Age ≥12 months (n=278) | 92 | 186 | ||
| Side of the lesion: bilateral (n=252) | 69 | 183 | 0.049 | 0.976 |
| Side of the lesion: left (n=162) | 43 | 119 | ||
| Side of the lesion: right (n=105) | 29 | 76 | ||
| Male (n=334) | 84 | 250 | 1.653 | 0.199 |
| Female (n=185) | 57 | 128 | ||
| Fever (n=75) | 10 | 65 | 7.682 | 0.006* |
| No fever (n=444) | 131 | 313 | ||
| Previous UTI in the last 3 months (n=241) | 39 | 202 | 26.412 | 0.000* |
| No UTI in the last 3 months (n=278) | 102 | 176 | ||
| Previous antibiotics administration in the last 3 months (n=285) | 41 | 244 | 50.768 | 0.000* |
| No antibiotics administration in the last 3 months (n=234) | 100 | 134 |
*, P<0.05. EBSL, extended-spectrum-β-lactamase; UTI, urinary tract infection.
Forward logistic regression analysis identified underlying neurologic disorder (OR =8, 95% CI: 1.845–34.695, P=0.005) and history of previous antibiotics administration in the last 3 months (OR =4.764, 95% CI: 3.114–7.289, P=0.000) as independent risk factors for ESBL positive UTI (Table 2).
Table 2
| Variable | OR (95% CI) | P value |
|---|---|---|
| Presence of underlying neurologic disorder | ||
| No | 1 | |
| Yes | 8 (1.845–34.695) | <0.01 |
| Previous antibiotics administration in the last 3 months | ||
| No | 1 | |
| Yes | 4.764 (3.114–7.289) | <0.01 |
95% CI, 95% confidence interval; OR, odds ratio; EBSL, extended-spectrum-β-lactamase; UTI, urinary tract infection.
Diagnostic use of nomogram
To predict the probability of ESBL (+) UTI, we also used multivariable logistic regression coefficients to construct a corresponding nomogram and calibration plot for these data (Figure 3). The nomogram generated was well calibrated for all predictions of ESBL+ probability, and the accuracy of the model nomogram measured by Harrell’s C statistic (C-index) was 0.741.
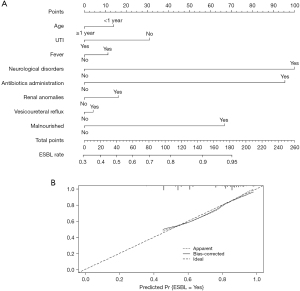
In the final points-based nomogram (Figure 3A), significant predictors are located on the left side, tailing with their respective scales on the right. Each scale position has corresponding points located on the “point” scale. The sum of all points for each variable was used to calculate the “Total Points”. Each “Total Points” represents the different probability to be a ESBL (+) UTI. For example, in a 3-months-old patient, who had previous UTI, fever, a history of antibiotics administration in the last 3 months, renal anomalies and vesicoureteral reflux. For this patient, the “Total Points” =14 (<12 months) + 0 (previous UTI) + 11 (fever) + 95 (antibiotics administration) + 16 (renal anomalies) + 4 (vesicoureteral reflux) = 140. The probability of ESBL (+) for this patient was 88.9%. The calibration plots (Figure 3B) suggested, in general, the nomogram was well calibrated for all predictions of ESBL (+) probability.
Discussion
This study describes trends in the microbial etiology of and antimicrobial resistance in UTI during 1994 and 2019 in the urology ward in the Chongqing Medical University Affiliated Children’s Hospital (Chongqing, China). Within this geographic area, we compared data on 854 urine samples from the pediatric population.
Extended spectrum beta-lactamases (ESBLs) are enzymes capable of hydrolysing penicillins, broad-spectrum cephalosporins and monobactams, and are generally derived from TEM and SHV-type enzymes. Antibiotics-resistant bacteria especially the ESBL-producing bacteria are increasing worldwide and are a serious problem in infection control (13,14). To our knowledge this is the first pediatric study from Chongqing that assess the risk factors for the emergence of ESBL-producing bacteria.
Although the prevalence of ESBLs is not known, it is clearly increasing. In our study of hospitalized patients with UTI over a 26 years interval 72.64% were ESBL producers which is a high percentage. Hanna-Wakim et al. (15) had evaluated hospitalized children over ten years and reported an ESBL positivity rate of 15.5%. A recent systematic review and metanalysis has shown a pooled prevalence of pediatric ESBL UTI of 5% in eastern Mediterranean studies compared to an overall prevalence of 14% in all other countries (4). However, it has reached to 46% and 49.3% in a cohort study from Turkey and Jordan (6,16). Considering all species together, we did find a statistically significant increase in ESBL-producing pathogens. There was also an observed increase in the proportion of ESBL producing pathogens among E. coli UTI. This high percentage and the trend could be attributed to the fact that our study included only hospitalized children in a third-grade class A hospital where more complicated cases were admitted. Furthermore, some of the patients were hospitalized and treated with antibiotics for a long period because of UTI before transferring to our hospital. The high proportion of children with an underlying urological abnormality would also promote ESBL presence increasing.
ESBL-producing strains are particularly important as they are resistant to all penicillin, to aztreonam and the majority cephalosporins (including third and fourth generation agents), furthermore, they are often cross-resistant to trimethoprim/sulfamethoxazole and quinolones. Thus, early identification of ESBL production is important in terms of appropriate treatment and effective infection control. While finding the risk factors of ESBL-producing would contribute to this item.
Previous studies have shown that hospitalization and use of antibiotics in the last 3 months, history of recurrent UTI, and presence of renal anomalies were important risk factors for ESBL-UTI (13). Neurological patients are increasingly vulnerable to a UTI due to the presence of a neurogenic bladder or maintenance of a urinary catheter. Previous studies also show the proportion of ESBL-producing UTI is increasing in neurological patients (11,17). However, whether neurologic disorder (e.g., tethered cord syndrome, meningitis, hypoxic ischemic encephalopathy, invisible spina bifida) and urodynamic change were risk factors for ESBL-UTI is still unknown. Studies also reported that the prevalence of UTI and ESBL (+) UTI was higher in malnourished children (13,14). Whether undernourishment was independent risk factors for ESBL positive UTI is also unclear. Hence, our study identified age at diagnosis as <12 months, fever, presence of urological malformation, vesicoureteral reflux, urodynamic change, neurologic disorder, undernourishment, previous use of antibiotics in the last 3 months and previous UTI in the last 3 months as predictors of acquisition of ESBL-UTI.
It is suggested that children with underlying neurologic disorder (e.g., tethered cord syndrome, meningitis, hypoxic ischemic encephalopathy, invisible spina bifida) was identified as the most significant risk factor by for ESBL-UTI in our study. This was supported by other studies performed on the risk factors for UTIs by ESBL-producing bacteria in neurological patients. Although they didn’t perform logistic regression analysis to identified neurological disorders as independent risk factors for ESBL positive UTI, there was an observed increase in the proportion of ESBL producing pathogens among children with neurological abnormality (11). Therefore, it is necessary to perform a detailed history-taking and physical examination among UTI children to identify the presence of underlying neurological disease.
Previous studies have identified previous antibiotic use and children with recurrent UTI as independent factors for ESBL UTI (18). This trend was also observed in our study. Forward logistic regression analysis identified history of previous antibiotics administration in the last 3 months as independent risk factors for ESBL positive UTI (OR =4.726, 95% CI: 3.090–7.229, P=0.000). Although the exact mechanisms underlying this association are not clear, we considered that previous antibiotics administration will lead to colonization of these children with ESBL organisms predisposing them to future UTI. This might be also explained by the increasing rate of fecal colonization with these resistant bacteria in healthy carriers (11,18).
In our cohort of patients, we observed a trend regarding a higher incidence of ESBL cases in children less than year (55.8% of ESBL cases) which is similar to two studies from Turkey and one study from Jordan (6). In our study, 40% of the children less than one year of age and 38.75% of those more than one year of age had ESBL producing bacteria that were resistant to amoxicillin/clavulanic in 58.70%, to nitrofurantoin in 22.62%, to quinolones in 43.14%, and gentamicin in 59.67%.
All children with known mechanical obstruction (posterior urethral valves, strictures) and functional obstruction (lower urinary tract dysfunction of either neurogenic or non-neurogenic origin and dilating vesicoureteral reflux) are considered to be related to ESBL (+) UTI (14,19). This trend was also observed in our study. Presence of congenital urological anomalies, presence of vesicoureteral reflux, fever and previous UTI in the last three months were statistically significantly associated with ESBL-UTI. Although these factors were not identified as independent risk factors for ESBL (+) UTI with conditional logistic regression analysis. These patients require hospitalization and parenteral antibiotics. Prompt anatomical evaluation of the urinary tract is critical to exclude the presence of significant urological or neurological abnormalities. If mechanical or functional abnormalities are present, adequate drainage of the infected urinary tract is necessary.
In a systematic review and meta-analysis, 26 cross-sectional and 8 case-control studies reporting on UTI prevalence in malnourished children was included, and they found that UTI is more prevalent in malnourished children than in their well-nourished counterparts (9). Another study included 402 malnourished children in Tanzania and data showed that more than 37% of UTI isolates were exhibiting extended spectrum beta lactamase (ESBL) phenotype (10). Although 83.33% of the nourished children were ESBL (+) in our study, this factor was not significantly associated with ESBL-UTI.
In the present series, there were no clinically significant factors as gender, side of the lesion and with urodynamic abnormality, in regards to the acquisition of ESBL-UTI in these infants. Thus, these data suggest three possible points of intervention: (I) the policy of restricted indications for administrating antibiotics could reduce the incidence of resistance; (II) optimise antibiotic use among young children with urological anomalies, vesicoureteral reflux and recurrent UTI; (III) choosing broad-spectrum antibiotics, including carbapenems, as empirical antibiotics for UTI among children with neurological disease and used antibiotic in the last 3 months.
Guidelines from the National Institute for Health and Clinical Excellence and American Academy of Pediatrics differ from each other in terms of the diagnostic algorithm to be followed. Treatment of ESBL-UTI and antibiotic prophylaxis for prevention of recurrent UTI are also areas of considerable debate. At present, the 2nd or 3rd generation cephalosporins and amoxicillin/clavulanic acid are recommended the first choice for empirical treatment (20). Some studies have demonstrated that once daily parenteral administration of gentamicin or ceftriaxone in a day treatment center is safe, effective and cost-effective in children with UTI (21,22). However, the major concern in our study is the high resistance to 2nd generation cephalosporins (>80%), 3rd generation cephalosporins (>80%) and the amoxicillin/clavulanic acid (>55%). Similarly, excluding amikacin, carbapenems, vancomycin and piperacillin/tazobactam, antibiotics with sensitivity higher than 50% to ESBL-producing pathogens were absent in our study.
Edlin et al. (23) found that although widely used, trimethoprim-sulfamethoxazole is a poor empirical choice for pediatric urinary tract infections in many areas due to high resistance rates. First-generation cephalosporins and nitrofurantoin are appropriate narrow-spectrum alternatives given their low resistance rates. In agreement with their study, nitrofurantoin is an appropriate choose with resistance rates <25%. Therefore, overconsumption in low-risk settings should be avoided and pediatricians should be aware about the specific treatment options. Any recommendation about (initial) antibiotic treatment should be regularly updated and adapted to local resistance profiles.
Notably, the proportion of patients with fever was 14.4% in our study. This proportion is lower than reported proportions for similar studies (12,21,24). The exact reason for this result is unknown. Over half of the patients (54.9%) had used antibiotics within 3 months of admission in our study. Patients may be afebrile at admission combined with the fact that patients are pre-treated with antibiotics. The reasons behind this phenomenon deserve further investigation.
This study has some limitations. We reviewed limited data collected from a single medical center, so it may not reflect the national status of microbial etiology of and antibiotics resistance in ESBL-UTI. However, this study is very important as no previous studies were characterizing long-term surveillance data for UTIs in all urological patients in a tertiary referral hospital in China. And since this was a retrospective study and depended on medical records, some UTI events may have been missed due to insufficient medical records. So, the nationwide surveillance program of microbial etiology and antibiotics resistance in infectious diseases is needed.
Conclusions
In conclusion, there is an increasing presence of ESBL-UTI and urologist should act timely. Children with underlying neurologic disorders and previous use of antibiotics are at increased risk for these infections. Identifying these risk factors and developing a nomogram for predictions of ESBL+ probability will greatly help us to guide empirical antimicrobial treatment while awaiting cultures.
Acknowledgments
We thank Medjaden Inc. for scientific editing of this manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81571425).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-21-523/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-21-523/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-523/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Board of Children’s Hospital of Chongqing Medical University (No. 22020278) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shaikh N, Mattoo TK, Keren R, et al. Early Antibiotic Treatment for Pediatric Febrile Urinary Tract Infection and Renal Scarring. JAMA Pediatr 2016;170:848-54. [Crossref] [PubMed]
- Zorc JJ, Kiddoo DA, Shaw KN. Diagnosis and management of pediatric urinary tract infections. Clin Microbiol Rev 2005;18:417-22. [Crossref] [PubMed]
- Moxon CA, Paulus S. Beta-lactamases in Enterobacteriaceae infections in children. J Infect 2016;72:S41-9. [Crossref] [PubMed]
- Flokas ME, Detsis M, Alevizakos M, et al. Prevalence of ESBL-producing Enterobacteriaceae in paediatric urinary tract infections: A systematic review and meta-analysis. J Infect 2016;73:547-57. [Crossref] [PubMed]
- Sorlozano A, Jimenez-Pacheco A, de Dios Luna Del Castillo J, et al. Evolution of the resistance to antibiotics of bacteria involved in urinary tract infections: a 7-year surveillance study. Am J Infect Control 2014;42:1033-8. [Crossref] [PubMed]
- Topaloglu R, Er I, Dogan BG, et al. Risk factors in community-acquired urinary tract infections caused by ESBL-producing bacteria in children. Pediatr Nephrol 2010;25:919-25. [Crossref] [PubMed]
- Dayan N, Dabbah H, Weissman I, et al. Urinary tract infections caused by community-acquired extended-spectrum β-lactamase-producing and nonproducing bacteria: a comparative study. J Pediatr 2013;163:1417-21. [Crossref] [PubMed]
- Megged O. Extended-spectrum β-lactamase-producing bacteria causing community-acquired urinary tract infections in children. Pediatr Nephrol 2014;29:1583-7. [Crossref] [PubMed]
- Uwaezuoke SN, Ndu IK, Eze IC. The prevalence and risk of urinary tract infection in malnourished children: a systematic review and meta-analysis. BMC Pediatr 2019;19:261. [Crossref] [PubMed]
- Ahmed M, Moremi N, Mirambo MM, et al. Multi-resistant gram negative enteric bacteria causing urinary tract infection among malnourished underfives admitted at a tertiary hospital, northwestern, Tanzania. Ital J Pediatr 2015;41:44. [Crossref] [PubMed]
- Shin HR, Moon J, Lee HS, et al. Increasing prevalence of antimicrobial resistance in urinary tract infections of neurological patients, Seoul, South Korea, 2007-2016. Int J Infect Dis 2019;84:109-15. [Crossref] [PubMed]
- Mori R, Lakhanpaul M, Verrier-Jones K. Diagnosis and management of urinary tract infection in children: summary of NICE guidance. BMJ 2007;335:395-7. [Crossref] [PubMed]
- Rupp ME, Fey PD. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs 2003;63:353-65. [Crossref] [PubMed]
- Bruchet N, Gaschignard J, Timsit S, et al. Risk of recurrence in children with a urinary tract infection due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. Acta Paediatr 2020;109:2808-9. [Crossref] [PubMed]
- Hanna-Wakim RH, Ghanem ST, El Helou MW, et al. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol 2015;5:45. [Crossref] [PubMed]
- Albaramki JH, Abdelghani T, Dalaeen A, et al. Urinary tract infection caused by extended-spectrum β-lactamase-producing bacteria: Risk factors and antibiotic resistance. Pediatr Int 2019;61:1127-32. [Crossref] [PubMed]
- Poisson SN, Johnston SC, Josephson SA. Urinary tract infections complicating stroke: mechanisms, consequences, and possible solutions. Stroke 2010;41:e180-4. [Crossref] [PubMed]
- Karam G, Chastre J, Wilcox MH, et al. Antibiotic strategies in the era of multidrug resistance. Crit Care 2016;20:136. [Crossref] [PubMed]
- Andreu A, Alós JI, Gobernado M, et al. Etiology and antimicrobial susceptibility among uropathogens causing community-acquired lower urinary tract infections: a nationwide surveillance study. Enferm Infecc Microbiol Clin 2005;23:4-9. [Crossref] [PubMed]
- Awais M, Rehman A, Baloch NU, et al. Evaluation and management of recurrent urinary tract infections in children: state of the art. Expert Rev Anti Infect Ther 2015;13:209-31. [Crossref] [PubMed]
- Doré-Bergeron MJ, Gauthier M, Chevalier I, et al. Urinary tract infections in 1- to 3-month-old infants: ambulatory treatment with intravenous antibiotics. Pediatrics 2009;124:16-22. [Crossref] [PubMed]
- Gauthier M, Chevalier I, Sterescu A, et al. Treatment of urinary tract infections among febrile young children with daily intravenous antibiotic therapy at a day treatment center. Pediatrics 2004;114:e469-76. [Crossref] [PubMed]
- Edlin RS, Shapiro DJ, Hersh AL, et al. Antibiotic resistance patterns of outpatient pediatric urinary tract infections. J Urol 2013;190:222-7. [Crossref] [PubMed]
- Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med 2011;365:239-50. [Crossref] [PubMed]


