
The role and mechanism of 1,25-dihydroxyvitamin D3 in regulating the Rho-kinase signaling pathway in asthmatic rats
Introduction
Bronchial asthma (referred to as asthma in the present study) is the most common chronic airway inflammatory disease in childhood (1). In 2010, the prevalence of asthma in urban children under 14 years old in China reached 3.02% (1). Hence, asthma seriously affects the physical and mental health of children (1). Asthma is mainly induced by the long-term infiltration of airways through a variety of cells. Among these cells, eosinophil (EOS) and neutrophil are the most infiltrative, which can release a variety of inflammatory mediators, damage bronchial epithelial cells and microvascular endothelial cells, and in turn, causes airway hyperresponsiveness (AHR) (2). In addition to inflammatory cells, airway structural cells, such as airway smooth muscle cells (ASMCs), can cause inflammation and airway remodeling by mediating inflammatory responses and releasing cytokines, chemokines and inflammatory mediators (3). Airway remodeling is the profound change and reorganization of respiratory tissues at the cellular and molecular level, which can lead to chronic asthma, persistent and irreversible airway obstruction, AHR, progressive lung damage, severe asthma and hormonal resistance (4).
The rhodopsin (Rho)/Rho-associated coiled forming protein kinase (ROCK) signaling pathway plays an important role in the pathophysiological process of asthma, and most of its family members regulate the assembly of the actin cytoskeleton, and are involved in cell migration (5). ROCK is widely found in many tissues and organs. This signaling pathway mediates airway smooth muscle (ASM) contraction, myofibroblast differentiation, and the maturation and migration of ASMCs and inflammatory cells, thereby playing an important role in chronic airway inflammatory diseases, such as asthma (6). A study revealed that the ROCK inhibitor Y27632 plays an important role in controlling T-helper cell-mediated inflammation, AHR, pro-inflammatory response, airway remodeling, and airway and alveolar septal oxidative stress in a mouse model of chronic allergic inflammation (2).
Glucocorticoid is the first choice of drugs for asthma prevention and treatment, which can effectively inhibit airway inflammation and the proliferation of ASMCs, and reduce AHR (7). Patients with severe or refractory asthma have poor treatment outcomes, despite adhering to high-dose inhaled corticosteroids, resulting in persistent or recurrent symptoms (8). Repeated exposure to allergens can cause glucocorticoid insensitivity in asthmatic mice (9). Its mechanism is associated with a decrease in the effectiveness of glucocorticoid receptors and the subsequent functional loss of steroids that regulate key regulatory proteins (3), as well as decreased compliance due to the side effects of glucocorticoids and misunderstandings of patients.
Therefore, in order to better improve the quality of life of asthma patients, the effective control of asthma to achieve clinical remission or complete control is the goal of the prevention and treatment of asthma. Vitamin D has exhibited anti-inflammatory and corticosteroid effects in both hormone-resistant and hormone-sensitive asthmatic patients (10). The 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] is an active metabolite of vitamin D. The present research team (4) found that 1,25-(OH)2D3 could reduce the production of eotaxin and IL-8 in alveolar lavage fluid and serum in rats with acute asthma, and inhibit the proliferation, migration and cell cycle progression of ASMCs. Hence, how is this effect achieved? How does 1,25-(OH)2D3 participate in the regulation of asthma? The present study explores these issues. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-365).
Methods
Materials
Healthy female specific-pathogen free (SPF) Wistar rats, weighing 130-150 g, were provided by the Animal Experimental Center of Xiamen University Medical College. Experiments were performed under a project license (No. XMULAC20160509) granted by the Animal Ethics Committee of Xiamen University, in compliance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals. At 1 week before the animal experiment, these rats were given an ovalbumin (OVA)-free diet. The OVA (grade V), 1,25-(OH)2D3 and dexamethasone (DXM) were purchased from Sigma (USA). The recombinant human TNF-α was purchased from Peprotech (USA). The eotaxin and IL-8 ELISA kits were purchased from R&D (USA). The rabbit anti-rat vitamin D receptor (VDR) antibody and rabbit anti-rat MLC20 antibody were purchased from Santa Cruz (USA). The rabbit anti-rat ROCK antibody was purchased from Sigma (USA). The rabbit anti-rat phosphorylated MLC20 antibody was purchased from Invitrogen (USA). The PCR primers were purchased from Shanghai Invitrogen Biotechnology Co., Ltd. The real-time quantitative PCR kit was purchased from Dalian TaKaRa Bio-Engineering Co., Ltd.
Methods
Establishment of the animal model
The asthma model was established according to the slightly modified Wistar rat asthma modeling method (5,6). On the first day, each rat in the asthma group was intraperitoneally injected with 1 ml of antigen-sensitizing solution (0.1% OVA, aluminum hydroxide, and 6×109 of inactivated Bordetella pertussis) for sensitization. On the 8th day, 1 mL of the mixture of OVA and aluminum hydroxide was intraperitoneally injected in rats. From the 15th day, these rats were placed in a self-designed closed container (20×20×20 cm), and allowed to inhale 1% OVA through ultrasonic nebulization for 30 minutes per time, once a day, for seven consecutive days.
Acquisition, purification and passage of cells
The above mentioned asthmatic rats were obtained and anesthetized with 10% chloral hydrate, the tracheas were obtained under sterile condition, the inner and outer membranes and cartilage were stripped, and the smooth muscle strips were cut into small pieces and digested with 0.1% trypsin and type-IV collagenase. Then, the ASMCs were purified by differential adherence, inoculated in a culture flask, and placed in an incubator at 37 °C. These cells were passaged when they grew to 80–90% confluence.
Cell grouping
ASMCs cultured to 4–6 generations and 80% confluence were digested with 0.25% trypsin, and transferred to a culture flask at a ratio of 1:2. When cells grew to 80% confluence, these cells were deprived of nutrients for 24 hours, allowing them to grow in synchronization. After 24 hours, these cells were divided into the following groups: control (N) group, the acute asthma model group; TNF-α (TNF) group; 1,25-(OH)2D3 (VD) group; DXM group; 1,25-(OH)2D3 + DXM (L) group. Cells in the control group were added with Dulbecco’s modified Eagle medium (DMEM) containing 0.1% bovine serum albumin (BSA). Cells in the TNF group were cultured in medium containing 10 ng/mL of TNF-α. The medium in the VD group contained 10-7 M of 1,25-(OH)2D3, the medium in the DXM group contained 10–7 M of DXM, and the medium in L group contained 10–7 M of 1,25-(OH)2D3 and 10–7 M of DXM. After the above treatments were performed for 1 hour, cells in the VD, DXM and L groups were added with TNF-α to a final concentration of 10 ng/mL. After 24 hours of culture, the supernatant was collected. Then, the cell suspension was centrifuged at 1,500 rpm for 10 minutes, and the supernatant and cells were separately placed in EP tubes and stored in a refrigerator at –80 °C. At the same time, these cells were treated and detected according to the requirements of the experiment.
Detection of eotaxin and IL-8 in the cell culture supernatant of asthmatic rats using enzyme-linked immunosorbent assay (ELISA)
Double antibody sandwich ELISA was performed. The samples were coated with anti-rat eotaxin and IL-8 monoclonal antibodies, and placed on the ELISA plate, in order to allow the eotaxin and IL-8 in the controls and samples to bind to the monoclonal antibodies. Then, these were added with anti-rat eotaxin and IL-8 antibodies to form an immune complex, and added with horseradish peroxidase-labeled streptavidin and biotin. Afterwards, these were allowed to bind with each other, and 3,3',5,5'-tetramethylbenzidine (TMB) was added for coloration. The optical density (OD) was measured at a wavelength of 450 nm. Since eotaxin and IL-8 concentrations are directly proportional to the D450 value, the concentration of eotaxin and IL-8 in the samples could be calculated by drawing a standard curve or direct reading.
Detection of VDR, ROCK and MLC20 mRNA levels in ASMCs by real-time quantitative PCR
The SYBR Green I fluorescent dye inlay method was used to draw the standard curves of the target genes and housekeeper gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. The standard curve was used to quantitatively analyze the target genes and housekeeping genes in the samples. The housekeeping gene was used to correct and detect the relative expression levels of target genes in each group.
Detection of VDR, ROCK, MLC20 and P-MLC20 protein levels in ASMCs of each group by western blot
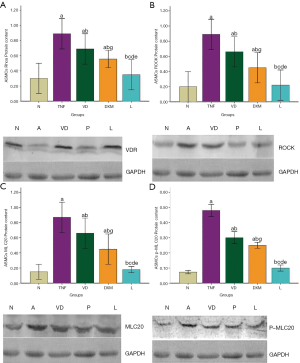
The frozen specimens were taken out, digested, cells were collected from all groups, and lysis and centrifugation were performed. Then, the proteins were extracted, diluted with lysis buffer, and 50 μL of protein from each sample was loaded. Afterwards, the supernatant underwent 12% polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, blocked with TBS containing 5% degreased milk powder, and left standing overnight at 4 °C. Next, the membrane was washed, added with the first antibody (1:400), incubated for 2 hours, added with the secondary antibody labeled with alkaline phosphatase (1:2,000), placed at room temperature for 2 hours, and washed and luminescenced. The experiment in each group was performed in triplicate, FlourChem V 2 Gel imaging analysis software was used to analyze the results, the gray values of all protein electrophoresis bands were recorded, and a quantitative analysis was performed. Protein content = sample protein gray value/the same sample β-actin gray value (Figures 1,2).
Statistical analysis
The data for the experiments in all groups were analyzed using statistical software SPSS 13.0. Measurement data were expressed as mean ± standard deviation (). When the variance was homogeneous, data were compared using one-way analysis of variance (ANOVA), and pairwise comparison was performed using Student-Newman-Keuls-q (SNK-q) test. When the variance was heterogeneous, the variables were compared using Kruskal-Wallis H test. P<0.05 was considered statistically significant.
Results
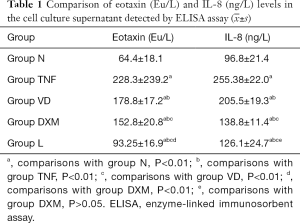
Comparison of eotaxin (Eu/L) and IL-8 (ng/L) levels in the cell culture supernatant detected by ELISA assay
Eotaxin level in the cell culture supernatant
The differences in eotaxin level in the cell culture supernatant between the N group and other groups were statistically significant (P<0.01). The eotaxin level in the cell culture supernatant was significantly higher in the TNF group, when compared to the N group and all treatment groups, and the differences were statistically significant (P<0.01). The eotaxin level was significantly lower in the VD group, when compared to the TNF group, but was significantly higher, when compared to the N and L groups, and the differences were highly statistically significant (P<0.01). Furthermore, the eotaxin level was higher, when compared to the DXM group, and the difference was statistically significant (P<0.05). The eotaxin level was significantly lower in the L group, when compared to the TNF, DXM and VD groups, and the differences were highly statistically significant (P<0.01). However, the eotaxin level was higher, when compared to the N group, and the difference was highly statistically significant (P<0.01, Table 1).
Full table
IL-8 level in the cell culture supernatant
The IL-8 level was significantly lower in the N group, when compared to the other groups, and the differences were statistically significant (P<0.01). The IL-8 level was significantly higher in the TNF group, when compared to the N group and all treatment groups, and the differences were statistically significant (P<0.01). The IL-8 level in the VD group was significantly lower, when compared to the TNF group (P<0.01), but this was significantly higher, when compared to the N, DXM and L groups, and the difference was statistically significant (P<0.01). The IL-8 level was higher in the L group, when compared to the N group, and the difference was statistically significant (P<0.01), but this was significantly lower than the TNF and VD groups, and the differences were statistically significant (P<0.01). However, the difference between the L and DXM groups was not statistically significant (P>0.05, Table 1).
Effect of ROCK and MLC20 mRNA levels in VDR and the Rho/ROCK signaling pathway in ASMCs of acute asthma model rats treated and cultured in vitro and detected by real-time PCR
Real-time PCR was used for the relative quantitative analysis, and the results revealed that the melting temperature of VDR-specific products in ASMCs of each group was 82.0 °C. After standardizing with the internal control GAPDH, the relative mRNA levels of all target genes (Table 2) were compared, and the results were as follows: VDR mRNA level: compared with the N group, the VDR mRNA level was significantly lower in the TNF group (P<0.01), while the mRNA level was significantly higher in the VD group (P<0.01), but the VDR mRNA level did not change in the DXM group (P>0.05); the VDR mRNA level was not significantly upregulated in the L group, when compared with the VD group (P>0.05). The VDR mRNA level was significantly higher in the L group, when compared with the TNF group (P<0.01).
The relative quantitative analysis by real-time quantitative PCR revealed that after the standardization of cells in all groups with the internal control GAPDH, the relative mRNA levels of target genes ROCK and MLC20 (Table 3) were compared. The statistical analysis results revealed that the mRNA levels of ROCK and MLC20 were significantly higher in the TNF group, when compared to all the other groups (P<0.01). The mRNA levels of ROCK and MLC20 were significantly downregulated in the VD group, and the differences between the VD group and the other groups were statistically significant, but the efficacy was weaker, when compared to that in the DXM group. After treatment, the differences in ROCK and MLC20 mRNA levels between the L group and N group were not statistically significant (P>0.05), the ROCK and MLC20 mRNA levels were significantly lower in the L group, when compared to the other groups (P<0.05), the efficacy in the L group was better than that in the VD or DXM group, and these two had a synergistic effect.
Detection of ROCK, MLC20 and P-MLC20 protein levels in VDR and the Rho/ROCK signaling pathway in ASMCs in each group by western blot
Western blot was used to detect the effect of intervention factors on the protein expression of VDR in ASMCs in each group. At the ASMC level, when compared with the N group, the VDR protein level was significantly lower in the group TNF (P<0.01), while the VDR protein level was significantly higher in the VD group (P<0.01). However, the protein level of VDR did not change in the DXM group (P>0.05). The protein level of VDR was not significantly upregulated in the L group, when compared with the VD group (P>0.05). The protein level of VDR was significantly higher in the L group, when compared with the TNF group (P<0.01, Figure 1). This result not only confirms the expression of VDR in ASMCs, but also reveals that 1,25(OH)2D3 could effectively upregulate the expression of VDR.
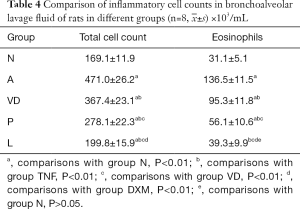
The protein levels of ROCK, MLC20 and P-MLC20 in the Rho/ROCK signaling pathway in ASMCs of acute asthma model treated and cultured in vitro in each group were detected by western blot at the ASMC level. The protein levels in ASMCs in rats in each intervention group were as follows: the levels of ROCK, MLC20 and P-MLC20 in the TNF group were significantly higher, when compared to the other groups (P<0.01). The levels of ROCK, MLC20 and P-MLC20 were significantly lower in the VD group, when compared to the TNF group (P<0.01). The levels of ROCK, MLC20 and P-MLC20 also significantly decreased in the DXM and L groups. The differences in the levels of ROCK and MLC20 between the L and N groups were not statistically significant (P>0.05), while the level of P-MLC20 was better in the L group, when compared to the VD and DXM groups (P<0.01) (Figure 1). Moreover, we compared the number of inflammatory cells in bronchoalveolar lavage fluid in different groups of rats (Table 4).
Full table
Discussion
Bronchial asthma is a heterogeneous disease characterized by chronic airway inflammation and AHR. Chronic airway inflammation is the basis for the onset, deterioration and persistence of asthma. ASMCs have strong growth and migration abilities, and both of these can lead to airway remodeling. The synthesis and secretion of chemokines and proliferation of ASMCs are enhanced in asthmatic individuals, which upregulate the concentration of local inflammation, and sustain and repeatedly aggravate inflammation, causing the contraction of AHR and ASM. The levels of inflammatory cells, cytokines and chemokines in asthma can be used as predictors of severity of asthma inflammation, asthma control and response to treatment, which are beneficial for the optimal treatment of asthma (7). Different types of chemokines have directional chemotaxis to specific inflammatory cells. Eotaxin and IL-8 are active components for the synthesis and secretion of ASMCs. Eotaxin is an important factor in asthma attacks, which can induce EOS, neutrophils and macrophages to migrate into the airways and be activated, and especially lead to the strong selective activation of EOS. EOS infiltration is correlated to airway epithelial injury, airway dysfunction, airway inflammation, airway reconstruction and AHR. Its count can be used as a predictor of acute exacerbation of asthma. IL-8 is a chemokine of EOS and neutrophils, which play a decisive role in asthma aggravation, severe asthma, refractory asthma and hormone-resistant asthma, and promote the occurrence of airway remodeling by increasing the migration and proliferation of ASMCs. Studies revealed that (8,9) in patients with severe resistant asthma, the level of IL-8 was significantly increased in sputum, and IL-8 could enhance the contraction of ASM by increasing the expression of L-shaped Ca2+. More interestingly (10), asthma, which is mainly characterized by neutrophil inflammation, has an increased bacterial load and a unique microbiota composition, and is accompanied by excessive inflammatory response. Therefore, understanding the relationship between airway microbiota and neutrophil inflammation would be helpful in the treatment and management of asthma.
The RhoA/Rho kinase signaling system mainly includes Rho and important downstream signaling molecules, such as ROCK, myosin light chain phosphatase (MLCP) and myosin light chain (MLC20), play an important role in actin cytoskeleton remodeling, cell migration and adhesion, contraction, proliferation and apoptosis, and the cell cycle regulation of ASMCs. Rho and Rho kinase have the Ca2+ sensitive contraction characteristic. This characteristic determines the contraction strength and degree of ASMCs. The classical substrates of Rho kinase are MLC20 and MLCP. The MLC phosphorylation level directly determines the contractile function of cells in ASMCs, and Rho kinase can accelerate and strengthen the contraction of smooth muscles by regulating its activity (11). A study revealed that (12) asthmatic patients often had irreversible or partially irreversible airflow obstruction and persistent AHR, and the cause may be the occurrence of airway remodeling, while the proliferation of ASMCs played an important role in airway remodeling. In the study of allergen sensitization and aggression-induced AHR model (13), the results revealed that the expression of RhoA increased, and that the Rho kinase inhibitor could effectively alleviate AHR. Therefore, the RhoA/Rho kinase signaling system can be used as an attractive target for asthma treatment.
As the first choice for the prevention and treatment of asthma, glucocorticoids can work in many patterns, including inhibiting the synthesis and release of inflammatory mediators, inhibiting chemotaxis, reducing vascular permeability, reducing tissue damage, blocking the synthesis of cytokines and chemokines, and effectively inhibiting the proliferation of ASMCs. Glucocorticoids can enter into the nucleus by binding to the corresponding receptors, in order to change the gene transcription, induce the apoptosis of EOS, and inhibit the migration of inflammatory cells to the airway, thereby improving AHR. However, there are statistically significant differences in therapeutic response to glucocorticoids among individuals, there are many side effects, and some asthmatic patients are insensitive or resistant to hormone therapy. Hence, the natural process of the disease cannot be changed (14). A study revealed that (15) the adverse effects of glucocorticoid inhalation therapy on CD4+ T cells in the internal and external tissues of the respiratory tract may not be safer than systemic therapy. In addition, the inhalation of glucocorticoids can negatively affect the presence of CD4+ T cells in the lungs. In addition, the patient’s misunderstanding leads to the reduction of compliance. Therefore, it is necessary to develop some substitute hormone drugs or drugs that have synergistic effects with hormones, or identify these drugs among existing drugs, in order to decrease the dosage of hormones.
Asthma is a polygenic hereditary disease. The VDR gene is a susceptible gene for asthma, which is widely distributed in various tissues and cells of the human body, and participates in the occurrence and development of asthma. The most classic role of vitamin D is to regulate calcium and phosphorus metabolism, and bone homeostasis. Furthermore, 1,25-(OH)2D3 is the most important active metabolite of vitamin D in vivo, which works by binding with VDR. Epidemiological studies have revealed that vitamin D deficiency and a susceptible population, especially patients with asthma and chronic obstructive pulmonary disease, rapid reduction in lung function, and increased inflammation, are correlated to the reduction of immunity (16). The treatment of asthma with vitamin D can reduce the incidence of respiratory tract infections (17,18), while respiratory tract infection can lead to the acute attack and aggravation of asthma. In patients with severe asthma, TH17 cytokine level is elevated, and not inhibited by steroids. However, 1,25-(OH)2D3 can inhibit the production of TH17 cytokines in all patients studied, and increase the number of Treg cells (19). Therefore, the new steroid enhancement property of vitamin D is conformed. In addition, the result revealed that the low serum level of vitamin D in asthmatic patients was correlated to the poor therapeutic effect of corticosteroids, and that vitamin D could enhance the effect of glucocorticoids in mononuclear cells in peripheral blood of asthmatic patients, and enhance the immunosuppressive function of DXM in vitro (20). Moreover, exposure to vitamin D in the process of fetal development can affect the immune system of newborns, thereby protecting newborns from asthma and other related diseases, including the impact of infectious diseases. From this, it can be observed that vitamin D plays an important role in individual development and immune defense.
In the present study, an ASMC primary culture was performed in vitro on the basis of the proliferation of ASMCs stimulated by TNF-α, 1,25(OH)2D3 and DXM treatment. These results revealed that the expression of chemokines eotaxin and IL-8 increased in the culture supernatant of ASMCs treated with TNF-α, and that 1,25-(OH)2D3 had obvious inhibition effect on this. Furthermore, the upregulated expression of VDR significantly downregulated the expression of ROCK, MLC20 and P-MLC20. The influences of DXM on the above factors were much greater than those of 1,25-(OH)2D3, and the inhibitory effect of the combination of these two drugs was more obvious, suggesting that these two has a synergistic effect.
In summary, asthma has obvious heterogeneity in clinical and molecular phenotypes. Hence, a specific targeted therapy is needed to block the key pathways of the disease. The RhoA/ROCK signaling pathway participates in the process of airway remodeling and AHR in asthma, while 1,25-(OH)2D3 may reduce airway inflammation by inhibiting chemokine production and inhibit the Rho/ROCK signaling pathway, participate in the regulation of asthma through this signaling pathway, and exhibit a synergic effect in combination with glucocorticoids. This is expected to help restore the sensitivity of glucocorticoids in asthma, and is considered to be one of the future drugs for asthma control (21).
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding: Science and Technology Project of Fujian Natural Science Foundation (2016J01615).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-365
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-365
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-365). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. XMULAC20160509) granted by the Animal Ethics Committee of Xiamen University, in compliance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cooperative Group on Childhood Asthma. Chinese Center for Disease Control and Prevention. Third nationwide survey of childhood asthma in urban areas of China. Zhonghua Er Ke Za Zhi 2013;51:729-35.
- Dos Santos TM, Righetti RF, Camargo LDN, et al. Effect of anti-IL17 antibody treatment alone and in combination with Rho-kinase inhibitor in a murine model of asthma. Front Physiol 2018;9:1183. [Crossref] [PubMed]
- Serra MF, Cotias AC, Pão CRR, et al. Repeated allergen exposure in A/J mice causes steroid-insensitive asthma via a defect in glucocorticoid receptor bioavailability. J Immunol 2018;201:851-60. [Crossref] [PubMed]
- Tian WM, Yang YG, Shang YX, et al. Role of 1,25-dihydroxyvitamin D3 in the treatment of asthma. Eur Rev Med Pharmacol Sci 2014;18:1762-9. [PubMed]
- Vanacker NJ, Palmans E, Kips JC, et al. Fluticasone inhibits but does not reverse allergen-induced structural airway changes. Am J Respir Crit Care Med 2001;163:674-9. [Crossref] [PubMed]
- Li M, Shang YX, Wei B, et al. The effect of substance P on asthmatic rat airway smooth muscle cell proliferation, migration, and cytoplasmic calcium concentration in vitro. J Inflamm (Lond) 2011;8:18. [Crossref] [PubMed]
- Meyer N, Nuss SJ, Rothe T, et al. Differential serum protein markers and the clinical severity of asthma. J Asthma Allergy 2014;7:67-75. [Crossref] [PubMed]
- Glenda E, Auteri S, Fabián C, et al. Significant increase of IL-8 sputum levels in treatment resistant severe asthma compared with difficult to treat severe asthma patients. J Genet Syndr Gene Ther 2014;5:218. [Crossref]
- Ding S, Zhang J, Yin S, et al. Inflammatory cytokines tumour necrosis factor-α and interleukin-8 enhance airway smooth muscle contraction by increasing L-type Ca2+ channel expression. Clin Exp Pharmacol Physiol 2019;46:56-64. [Crossref] [PubMed]
- Yang X, Li H, Ma Q, et al. Neutrophilic asthma is associated with increased airway bacterial burden and disordered community composition. Biomed Res Int 2018;2018:9230234 [Crossref] [PubMed]
- Shaifta Y, Irechukwu N, Prieto-Lloret J, et al. Divergent modulation of Rho-kinase and Ca(2+) influx pathways by Src family kinases and focal adhesion kinase in airway smooth muscle. Br J Pharmacol 2015;172:5265-80. [Crossref] [PubMed]
- Gosens R, Schaafsma D, Nelemans SA, et al. Rho-kinase as a drug target for the treatment of airway hyperrespon-siveness in asthma. Mini Rev Med Chem 2006;6:339-48. [Crossref] [PubMed]
- Chiba Y, Takada Y, Miyamoto S, et al. Augmented acetylcholine-induced, Rho-mediated Ca2+ sensitization of bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Br J Pharmacol 1999;127:597-600. [Crossref] [PubMed]
- Subspecialty Group of Respiratory Diseases, Society of Pediatrics, Chinese Medical Association. Editorial Board, Chinese Journal of Pediatrics. Guideline for the diagnosis and optimal management of asthma in children(2016). Zhonghua Er Ke Za Zhi 2016;54:167-81.
- Zuśka-Prot M, Maślanka T. Effect of inhaled and systemic glucocorticoid treatment on CD4+ regulatory and effector T cells in a mouse model of allergic asthma. Int Immunopharmacol 2017;45:98-109. [Crossref] [PubMed]
- Liu PT, Stenger S, Tang DH, et al. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 2007;179:2060-3. [Crossref] [PubMed]
- Ramos-Martínez E, López-Vancell MR, Fernández de Córdova-Aguirre JC, et al. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNγ levels and cathelicidin expression. Cytokine 2018;108:239-46. [Crossref] [PubMed]
- Fawaz L, Mrad MF, Kazan JM, et al. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol 2016;166-167:59-71. [Crossref] [PubMed]
- Şıklar Z, Karataş D, Doğu F, et al. Regulatory T cells and vitamin D status in children with chronic autoimmune thyroiditis. J Clin Res Pediatr Endocrinol 2016;8:276-81. [Crossref] [PubMed]
- Searing DA, Zhang Y, Murphy JR, et al. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 2010;125:995-1000. [Crossref] [PubMed]
- Pigati PA, Righetti RF, Possa SS, et al. Y-27632 is associated with corticosteroid-potentiated control of pulmonary remodeling and inflammation in guinea pigs with chronic allergic inflammation. BMC Pulm Med 2015;15:85. [Crossref] [PubMed]




