
Associations between WTAP gene polymorphisms and neuroblastoma susceptibility in Chinese children
Introduction
Neuroblastoma is a extracranial solid tumor derived from neural crest tissues, accounting for about 15% of pediatric tumor-related mortality (1). As one of the most common solid malignancies in children, neuroblastoma exhibits diverse clinical behaviors. The survival rate for patients with high-risk tumors is lower than 50% even after receiving multimodality treatment, while some patients undergo spontaneous regression after mild or no treatment (1,2). The pathogenesis of neuroblastoma is multifactorial and remains far from clear. Emerging evidence shows that the transformation from normal cells to tumor cells is attributed to a gradual accumulation of genetic alterations (2-4). It is imperative to reveal the genetic mechanisms of neuroblastoma formation, which has the potential to provide novel therapeutic approaches for refractory neuroblastoma. Advances in genome-wide association studies (GWASs) allow the detection of genetic variations in tumor samples and result in significant progress in the understanding of the heritability of neuroblastoma (4,5). At present, many genetic and epigenetic variations that not only contribute to tumorigenesis but also promote the malignant potential of neuroblastoma have been demonstrated by GWASs (6-8). Single nucleotide polymorphisms (SNPs) within HSD17B12, DDX4, and DUSP12 are enriched in patients with low-risk neuroblastoma (4,5,9). SNPs in LMO1, CASC15, and LIN28B are significantly correlated with high-risk neuroblastoma and are involved in promoting proliferation and invasion (8,10,11).
Wilms’ tumor 1-associating protein (WTAP), located at chromosome region 6q25-27, is involved in regulating embryonic development, cell proliferation and apoptosis (12,13). WTAP has also been identified as an oncogenic protein in diffuse large B-cell lymphoma and acute myeloid leukemia (14,15). Moreover, accumulating evidence indicates that WTAP plays an important role in the initiation and development of various human malignancies, including glioma, ovarian cancer, renal cell carcinoma and pancreatic ductal adenocarcinoma (16-18). The role of WTAP SNPs on the cancer susceptibility also has been investigated. Our research group have identified a significant relationship between rs7766006 and hepatoblastoma risk in the Chinese population (19). However, no study has been reported to evaluate the associations between WTAP SNPs and neuroblastoma susceptibility.
To assess the associations between the SNPs in WTAP and neuroblastoma risk, we carried out this case-control study of 898 neuroblastoma patients and 1,734 control subjects using a Chinese population of children. We present the following article/case in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/tp-20-168).
Methods
Study subjects
Here, we totally enrolled 898 neuroblastoma patients and 1,734 controls from eight hospitals from eight cities (Guangzhou, Zhengzhou, Wenzhou, Xi’an, Taiyuan, Kunming, Changsha, Shenyang) in China (Table S1). All the enrolled subjects were genetically unrelated and of Chinese descents. Age, sex, and ethnicity were well matched in the patients and controls. Neuroblastoma patients were diagnosed by biopsy and staged based on the International Neuroblastoma Staging System (INSS) (20). Each participant’s parents or guardians provided written informed consent. This study was approved by the Institutional Review Board of Guangzhou Women and Children’s Medical Center (No: 201929300). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Polymorphism selection and genotyping
Potential functional polymorphisms in the WTAP gene were searched in the dbSNP database (http://www.ncbi.nlm.nih.gov/) and SNPinfo (http://snpinfo.niehs.nih.gov/) according to the selection criteria described in our reported publication (21,22). Three SNPs (rs9457712 G>A, rs1853259 A>G and rs7766006 G>T) in the WTAP gene were eventually selected (23). These SNPs were detected by standard TaqMan real-time PCR (24-26). To assure the accuracy of genotyping results, 10% of the samples were selected randomly to run a second genotype. All repeated samples were 100% concordant.
Statistical analysis
Differences in genotype distribution and demographic characteristics between patients and controls were compared by two-sided χ2 tests. Hardy-Weinberg equilibrium (HWE) for the selected SNPs in controls was assessed by a goodness-of-fit χ2 test. Associations between neuroblastoma susceptibility and WTAP SNPs were evaluated using odds ratios (ORs) and 95% confidence intervals (CIs). Stratified analysis was conducted regarding age, sex, tumor sites, and clinical stages. P<0.05 was considered statistically significant. All statistical analyses were carried out using SAS software (Version 9.4; SAS Institute, Cary, NC, USA).
Results
WTAP gene polymorphisms and neuroblastoma susceptibility
In the current study, 896 cases and 1,732 controls were successfully genotyped. The genotype distribution of the three WTAP polymorphisms and their associations with neuroblastoma susceptibility are revealed in Table 1. All these SNPs were in accordance with HWE among the control subjects (P=0.213 for the rs9457712 G>A polymorphism, P=0.185 for the rs1853259 A>G polymorphism, and P=0.799 for the rs7766006 G>T polymorphism). No significant associations were detected between the selected WTAP SNPs and neuroblastoma susceptibility.
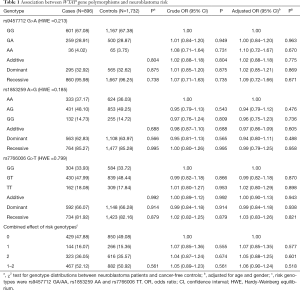
Full table
Stratification analysis
We further divided participants into subgroups based on sex, age, sites of tumor origin, and clinical stages. The effects of the selected SNPs on neuroblastoma risk were determined in this stratified analysis (Table 2). Our results indicated that children ≤18 months old with rs1853259 AG/GG genotypes were less likely to develop neuroblastoma (OR =0.77, 95% CI: 0.59–0.998, P=0.048). However, children ≤18 months old harboring 1-2 combined risk genotypes had increased neuroblastoma susceptibility (OR =1.32, 95% CI: 1.02–1.71, P=0.036).

Full table
Discussion
We performed this eight-center study to investigate the association between WTAP gene polymorphisms and neuroblastoma susceptibility. Our data manifested that rs1853259 AG/GG genotypes are correlated with a decreased neuroblastoma risk in children ≤18 months old. However, children ≤18 months old harboring 1–2 combined risk genotypes are more likely to develop neuroblastoma. To our knowledge, the current study represents the first to explore the association between WTAP SNPs and neuroblastoma susceptibility.
WTAP was initially identified as a nuclear protein and is involved in N6-methyladenosine RNA modification, which affects the initiation and progression of several human malignancies by modulating the mRNA expression of oncogene genes (12,27-29). In addition, WTAP can also execute oncogenic effects by inhibiting apoptosis, accelerating proliferation and promoting invasion of malignant cells (12,14,30). Previous studies have demonstrated that overexpression of WTAP is associated with poor survival in renal cell carcinoma, gastric cancer and pancreatic ductal adenocarcinoma (31-33).
Given the vital role of WTAP in the initiation and progression of malignancies, investigation into the association between WTAP SNPs and neuroblastoma susceptibility is warranted. Therefore, we conducted this study to explore the association between WTAP SNPs and neuroblastoma risk in Chinese children. In the current study, no significant associations were discovered in the overall analysis between the selected WTAP SNPs and neuroblastoma susceptibility. However, in the age ≤18 months subgroup, we found that rs1853259 AG/GG genotypes exerted protective effects against neuroblastoma, whereas the presence of 1–2 combined risk genotypes significantly increased the risk of neuroblastoma.
There are several limitations present in our current study. First, neuroblastoma is a remarkably heterogeneous disease with a complex etiology. However, several confounding factors, including dietary intake and living environment, were not assessed in our current study. The results should be explained with caution in the absence of other confounding factors. Further comprehensive study incorporating the combined analysis of genetic factors and confounding factors are warranted. Second, here we only analyzed three WTAP SNPs. Further investigation will be required to uncover more polymorphisms that predispose patients to neuroblastoma, which may provide novel insights into the genetic etiology of neuroblastoma. Third, ethnic background may affect genetic predisposition. Our results based on Chinese populations may not be directly extrapolated to other ethnicities. Fourth, the negative results might be attributed to the relatively small sample size in our current study, which might not be large enough to detect an association.
In summary, our study found that none of the WTAP polymorphisms (rs9457712 G>A, rs1853259 A>G and rs7766006 G>T) were related to neuroblastoma susceptibility in the overall analysis. The effect of WTAP SNPs on neuroblastoma predisposition must be elucidated by well-designed studies.
Acknowledgments
Funding: This study was supported by grants from the Natural Science Foundation of Guangdong Province (No: 2019A1515010360), the National Natural Science Foundation of China (No: 81560262, 81960294), and Guangdong Provincial Key Laboratory of Research in Structural Birth Defect Disease (No: 2019B030301004).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-168
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-168
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-168). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was acquired from the parents or guardians of each participant. This study was approved by the Institutional Review Board of Guangzhou Women and Children’s Medical Center (No: 201929300).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maris JM. Recent advances in neuroblastoma. N Engl J Med 2010;362:2202-11. [Crossref] [PubMed]
- Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers 2016;2:16078. [Crossref] [PubMed]
- Jordan EJ, Patil K, Suresh K, et al. Computational algorithms for in silico profiling of activating mutations in cancer. Cell Mol Life Sci 2019;76:2663-79. [Crossref] [PubMed]
- Bagatell R, Cohn SL. Genetic discoveries and treatment advances in neuroblastoma. Curr Opin Pediatr 2016;28:19-25. [Crossref] [PubMed]
- Tolbert VP, Coggins GE, Maris JM. Genetic susceptibility to neuroblastoma. Curr Opin Genet Dev 2017;42:81-90. [Crossref] [PubMed]
- Bosse KR, Diskin SJ, Cole KA, et al. Common variation at BARD1 results in the expression of an oncogenic isoform that influences neuroblastoma susceptibility and oncogenicity. Cancer Res 2012;72:2068-78. [Crossref] [PubMed]
- Russell MR, Penikis A, Oldridge DA, et al. CASC15-S Is a Tumor Suppressor lncRNA at the 6p22 Neuroblastoma Susceptibility Locus. Cancer Res 2015;75:3155-66. [Crossref] [PubMed]
- Wang K, Diskin SJ, Zhang H, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature 2011;469:216-20. [Crossref] [PubMed]
- Nguyen B, Diskin SJ, Capasso M, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet 2011;7:e1002026. [Crossref] [PubMed]
- Maris JM, Mosse YP, Bradfield JP, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med 2008;358:2585-93. [Crossref] [PubMed]
- Diskin SJ, Capasso M, Schnepp RW, et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet 2012;44:1126-30. [Crossref] [PubMed]
- Xie W, Wei L, Guo J, et al. Physiological functions of Wilms' tumor 1-associating protein and its role in tumourigenesis. J Cell Biochem 2019. Epub ahead of print. [Crossref] [PubMed]
- Little NA, Hastie ND, Davies RC. Identification of WTAP, a novel Wilms' tumour 1-associating protein. Hum Mol Genet 2000;9:2231-9. [Crossref] [PubMed]
- Kuai Y, Gong X, Ding L, et al. Wilms' tumor 1-associating protein plays an aggressive role in diffuse large B-cell lymphoma and forms a complex with BCL6 via Hsp90. Cell Commun Signal 2018;16:50. [Crossref] [PubMed]
- Bansal H, Yihua Q, Iyer SP, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia 2014;28:1171-4. [Crossref] [PubMed]
- Tang J, Wang F, Cheng G, et al. Wilms' tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J Exp Clin Cancer Res 2018;37:40. [Crossref] [PubMed]
- Yu HL, Ma XD, Tong JF, et al. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Ther 2019;12:6191-201. [Crossref] [PubMed]
- Jin DI, Lee SW, Han ME, et al. Expression and roles of Wilms' tumor 1-associating protein in glioblastoma. Cancer Sci 2012;103:2102-9. [Crossref] [PubMed]
- Wang Z, Cheng H, Xu H, et al. A five-gene signature derived from m6A regulators to improve prognosis prediction of neuroblastoma. Cancer Biomark 2020;28:275-84. [Crossref] [PubMed]
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11:1466-77. [Crossref] [PubMed]
- He J, Wang F, Zhu J, et al. Association of potentially functional variants in the XPG gene with neuroblastoma risk in a Chinese population. J Cell Mol Med 2016;20:1481-90. [Crossref] [PubMed]
- He J, Yang T, Zhang R, et al. Potentially functional polymorphisms in the LIN28B gene contribute to neuroblastoma susceptibility in Chinese children. J Cell Mol Med 2016;20:1534-41. [Crossref] [PubMed]
- Ma L, Hua RX, Lin H, et al. The contribution of WTAP gene variants to Wilms tumor susceptibility. Gene 2020;754:144839. [Crossref] [PubMed]
- He J, Qiu LX, Wang MY, et al. Polymorphisms in the XPG gene and risk of gastric cancer in Chinese populations. Hum Genet 2012;131:1235-44. [Crossref] [PubMed]
- He J, Zou Y, Liu X, et al. Association of Common Genetic Variants in Pre-microRNAs and Neuroblastoma Susceptibility: A Two-Center Study in Chinese Children. Mol Ther Nucleic Acids 2018;11:1-8. [Crossref] [PubMed]
- Chen X, Wang Y, Chen X, et al. Genetic variants in the regulatory region of SLC10A1 are not associated with the risk of hepatitis B virus infection and clearance. Infect Genet Evol 2016;44:495-500. [Crossref] [PubMed]
- Galardi S, Michienzi A, Ciafrè SA. Insights into the Regulatory Role of m(6)A Epitranscriptome in Glioblastoma. Int J Mol Sci 2020;21:2816. [Crossref] [PubMed]
- Deng X, Su R, Weng H, et al. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res 2018;28:507-17. [Crossref] [PubMed]
- Chen Y, Peng C, Chen J, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer 2019;18:127. [Crossref] [PubMed]
- Jo HJ, Shim HE, Han ME, et al. WTAP regulates migration and invasion of cholangiocarcinoma cells. J Gastroenterol 2013;48:1271-82. [Crossref] [PubMed]
- Li BQ, Huang S, Shao QQ, et al. WT1-associated protein is a novel prognostic factor in pancreatic ductal adenocarcinoma. Oncol Lett 2017;13:2531-8. [Crossref] [PubMed]
- Xi Z, Xue Y, Zheng J, et al. WTAP Expression Predicts Poor Prognosis in Malignant Glioma Patients. J Mol Neurosci 2016;60:131-6. [Crossref] [PubMed]
- Li H, Su Q, Li B, et al. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J Cell Mol Med 2020;24:4452-65. [Crossref] [PubMed]


