Management of children after renal transplantation: highlights for general pediatricians
Introduction
Renal transplantation is accepted as the treatment choice for children with end stage renal disease, as it provides better quality of life and improves long-term survival than other types of renal replacement therapies (1). According to the United Network for Organ Sharing (UNOS) database, over 16,900 renal transplantations were performed in children less than 18 between January 1988 and August 2011. Survival rates have been noted to improve during this time (2). Coinciding with this, immunosuppressive regimens and anti-rejection strategies in children have undergone dramatic changes over the last decade, as more potent drugs are now available to transplant nephrologists. As the nephrology teams at transplant centers are not in the best positions to provide primary care, optimal medical care for the transplant recipients require the collaborative efforts shared by all health care providers. Pediatricians are essential health care providers and their support becomes increasingly significant and pivotal following the acute phase of renal transplantation. Hence, it is vital for pediatricians to keep abreast of their knowledge regarding the special needs of children post renal transplantation.
Health maintenance care issues
Potential drug interactions with immunosuppressive therapies
Children require prolonged therapy with immunosuppressive drugs after transplantation and the short and long-term adverse effects associated with these agents remain a challenge for clinicians. Complicating this issue is the fact that children often require prescriptions for various clinical conditions and it becomes crucial for pediatricians to be informed about potential drug interactions when prescribing additional medications.
Most centers have started to use either polyclonal or monoclonal antibodies for induction therapy during the last 2 decades and newer and more potent antibodies are also being developed (3,4). Although complications and adverse effects of these antibodies exist, most of the children are not on any induction therapy when they are released back to their local community. However, it is very likely that the patients are on maintenance immunosuppression therapies (5,6). Until recently, post renal transplantation immunosuppressive therapies were usually comprised of a “triple drug combinations” of corticosteroids, a calcineurin inhibitor (cyclosporine or tacrolimus) and a DNA synthesis inhibitor (azathioprine or mycophenolate mofetil). As a point of note, the popularity of cyclosporine and azathioprine has diminished significantly during the last decade (4). Sirolimus, once quite popular in transplant community as the hopeful candidate for calcineurin inhibitor sparing agent, is being used less frequently now because of the high association of surgical complications such as lymphoceles and poor wound healing (7). In addition, the recent concerns on the potential effect on reducing spermatogenesis in male patients have also discouraged its use in some transplant centers (8). According to the 2010 annual report from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS), most of the children at 30 days after their renal transplantation are on combination of prednisone, tacrolimus and mycophenolate mofetil, with only a very minor proportion of them are being on either cyclosporine, azathioprine or sirolimus (9).
Although corticosteroids are the main drug used for anti-rejection, its use has been undesirable to most transplant nephrologists due to the significant side effects. More recently, there are successful reports from different centers in eliminating corticosteroids from their immunosuppressive regimen (10-13). Even though more and more pediatric transplant centers are now switching to steroid minimization protocols, there still exist concerns about the safety of such strategy, such as the more pronounced marrow suppressive effects of mycophenolate mofetil in the absence of corticosteroids (11,14). Early experiences suggest higher incidence of leucopenia and anemia in children receiving steroid avoidance or minimization therapies, and some children require granulocyte colony stimulating factor (GCSF) and erythropoietin treatment. Newer immunosuppressive agents are also being developed. One of the more promising drugs is alemtuzumab (Campath-1H, an anti-CD52), a new anti-T cell agent with prolonged and potent lymphocyte depletion effects after renal transplantation (15). Although its use in adult populations is promising, it is not widely used pediatric population at present.
Nonetheless, children are often prescribed medications and it is essential for pediatricians to be knowledgeable about the potential interferences of many drugs on the therapeutic levels of the immunosuppressants. Since cyclosporine and tacrolimus are metabolized in the liver by the cytochrome P450 IIIA systems, drugs that induce or inhibit this enzyme system will alter the blood levels.
Table 1 depicts the common drugs that interfere with calcineurin inhibitors used in children after renal transplantation. Of note, grape fruit, is contraindicated in patients receiving tacrolimus as it increases the plasma level (17). There are also other commonly used drugs that may potentially increase the risk of nephrotoxicity if given concurrently with the calcineurin inhibitors. Such medications include other nephrotoxic agents such as aminoglycosides, non-steroidal anti-inflammatory drugs, amphotericins, ciprofloxacin and intravenous contrast medium (16,18). Antacids such as aluminum magnesium milk may inhibit the absorption of calcineurin inhibitors and patients should be advised to take these medications separately. If in doubt, the physician should contact the transplant center before prescribing.
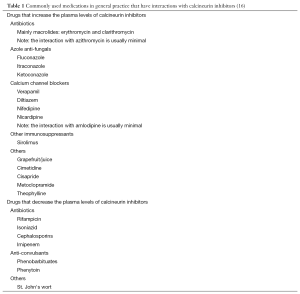
Full table
Fever in children with renal allograft
The etiologies of fever in children on immunosuppressive therapies are very diverse and the diagnosis may not be obvious. In a study from Minnesota, among renal allograft recipients with ages over 13, who were hospitalized due to fever, the most common causes were viral, bacterial and fungal infections, followed by allograft rejection, malignancy and drug fever (19). The management of fever should be tailored to the individual situation.
Dealing with infections in renal allograft recipients
Post transplantation infection remains one of the major causes of morbidity and mortality. The incidence of infection during the first 3 years after renal transplantation among adults was estimated to be 45 per 100 patient-years (20) and severe infections were responsible for 16% of mortality after renal transplantation (21). Although infection can occur at any time, children are most vulnerable to severe bacterial and viral infections during the first 3 to 6 months (20). According to the 2010 NAPRTCS Annual Report, during the first 5 months after renal transplantation, 45.6% of children with live donor transplantation were readmitted to the hospital with bacterial (11.7%), viral (12.3%) and fungal (0.7%) infections. Similar rates were also observed among children with deceased donor renal transplantations (22).
Bacterial infections
Bacterial infections are most commonly encountered in pediatric renal allograft recipients with preexisting chronic illnesses, and depending on the severity, may need to be admitted and managed by the nephrology team as inpatients.
Among children who suffer from bacterial infections, wound and urinary tract infections are the leading causes of re-admission after transplantation (23-26). Despite the higher incidence of wound complications among patients receiving sirolimus, the overall incidence of wound infections has been improving, due to a combination of better surgical techniques and judicious use of antibiotic prophylaxis (27). Urinary tract infections, however, are still frequent, varying from 30% to 40% among patients after renal transplantation, especially in presence ureteral stents (28-30). Trimethoprim-sulfamethoxazole (TMP- SMX) is usually given during the first 3 to 6 months as prophylaxis against pneumocystis pneumonia. There is limited data on the efficacy of TMP-SMX preventing other bacterial infections.
Pneumocystis pneumonia
Pneumocystis pneumonia is a serious opportunistic infection caused by Pneumocystis jirovecii. The nomenclature Pneumocystis carinii is not used anymore as this particular species is found only in rats. Patients with pneumocystis pneumonia may present with fever, non-productive cough, and constitutional symptoms such as weight loss and night sweat. It is rarely seen now after TMX-SMX prophylaxis is used routinely in all transplant recipients (31).
Viral infections related to immunosuppression
Transplant recipients are also prone to opportunistic infections due to their immunosuppressed state. As the presentation of viral infections can be protean, children with persistent and unexplained fever should be referred back to the transplant team for accurate diagnosis and prompt treatment.
Cytomegalovirus (CMV) infections
CMV is the most common viral cause of infection in transplant recipients and systemic diseases such as colitis have been reported in up to half of those affected (32). It may either be a primary infection acquired from the donor kidney or due to reactivation of latent disease. The most common symptoms include prolonged fever and other constitutional symptoms. Patients may complain of general malaise, poor appetite and musculoskeletal pain. Laboratory investigations are characterized by leucopenia, atypical lymphocytosis and thrombocytopenia. Diagnosis is usually confirmed by elevated CMV titers detected by polymerase chain reaction (PCR). The incidence and severity of CMV diseases have dramatically decreased over the last few decades due to the availability of more potent antivirals such as valganciclovir and incorporation of hyperimmuniglobulins (CytoGam) prophylaxis in high risk groups after transplantation (33).
Epstein barr virus (EBV) infections
The severity of EBV infection in children after transplantation may range from mild symptoms that resemble infectious mononucleosis, to much more severe illness including hepatitis and post-transplant lymphoproliferative diseases (PTLD). Potential risk factors for PTLD include young age at transplantation; non-immune recipient receiving an allograft from an EBV positive donor and multiple episodes of acute rejections required aggressive immunosuppression (34). Lymphadenopathy may be present but extra nodal presentation occurs in up to half of cases. Hence, EBV infection should be on the list of differential diagnosis in any children after renal transplantation with fever, especially when it is associated with lymphadenopathy and/or exudative tonsillitis. Diagnosis is further suggested by an increase in the viral titer by PCR. The diagnosis usually requires histological and immunofluorescence examination of tissue. It is common practice to reduce the dose of immunosuppression as the first line of treatment, then supplement with chemotherapy if necessary (35,36).
Polyoma (BK and JC) virus infection
BK and JC viruses are members of the polyoma virus family. Both viruses were named after the patients from whom the virus was first isolated. They are rarely pathogenic in immunocompetent individuals but may progress to nephropathy in allograft recipients. Affected patients may present with ureteral stenosis, interstitial nephritis and progressive loss of renal functions. Diagnosis is suggested by an increase in viral titer by PCR in blood and confirmed by renal biopsy (37).
Community acquired viral infection
After resuming school and normal social life, children on immunosuppressive therapy are frequently exposed to common pathogens such as influenza, respiratory syncytial virus and rotavirus. These often mild illnesses may become much more severe in children who are still on high dose of immunosuppressive therapies. Varicella-zoster virus infection may cause fulminant diseases in children without immunity. In the past, it was recommended that varicella-zoster immune globulin (VZIG) be administered within 72 hours of exposure in order to minimize the consequences. However, since VZIG may not be available, it is a reasonable alternative to empirically prescribe antiviral such as acyclovir or valganciclovir for a total of 3 weeks after exposure (38,39). All children should be monitored closely after exposure whether or not given antiviral therapy.
In brief, as most of the children are staying close to or being followed closely by the transplant team immediately after transplantation, the primary care physicians may not need to deal with early infections. After the acute phase, primary care physicians become the valuable resources for the family, especially for community acquired infections during peak viral seasons. However, if an obvious source of fever is not readily identifiable or CMV/EBV infections are suspected, the patient should be referred back to the transplant team for further evaluations. At our center, we usually admit all patients with temperature higher than 38.5 °C for observation when no clearly identifiable source found. If the patient has an obvious source of infection, such as a urinary tract infection, we may consider outpatient therapy if the family is living close to the hospital and has ready access to the transplant team.
Fever and rejection
Hyperacute rejection occurs when allograft recipients have pre-existing antibodies against the donors and it occurs soon after the transplantation, making it is unlikely that the primary care physician will need to deal with this catastrophe. Fortunately, the incidence of acute rejections has been declining steadily over the years. The rejection rate was over 70% between the years 1987 to 1990, and was down to 13% in the years 2007 to 2010 (22). Therefore, it is not unusual to see children to be rejection free during the first year after transplantation, but acute rejection can still happen later and patients may present initially to their primary care physicians with early signs of rejection anytime up to years.
However, while the possibility of rejection should be considered as part of the pediatrician’s differential diagnosis in the evaluation of febrile children with recent renal transplantation, the typical clinical features of rejections such as fever; graft tenderness and acute reduction in urine output are no longer seen commonly in clinical practice (40). Instead, most children with acute rejections have an acute rise in serum creatinine and renal biopsy is required in most instances to confirm the diagnosis. Some children may have subclinical rejections that will only become apparent on biopsy (41). Hence, when there is any suspicion, the patients should be referred back to the transplant centers immediately.
Chronic allograft rejection is now the leading cause of graft loss among children and it may occur any time after renal transplantation. Current literature indicates that repeat episodes of acute rejection and ongoing injury (secondary to insufficient or over immunosuppression) are independent risk factors for development of chronic rejection (42). Subtle signs such as swelling of lower limbs, easy fatigue, weight gain, worsening of hypertension and increase in proteinuria may be present. However, most patients do not have any symptoms except slow deterioration of the allograft function.
Although the care for children who suffer from either acute or chronic rejection remains the responsibility of the transplant team, the patients may present themselves initially to their pediatricians. Familiarity with the presentations and high index of suspicions are keys to early referral back to the transplant team.
Immunizations
Vaccination can prevent significant morbidity and mortality in children who receive long-term immunosuppression. However, timing of the vaccinations is important. For details in the current recommendation, please refer to the immunization schedules and guidelines suggested by the American Society of Transplantation (AST) (43). Although the antibody responses after vaccinations may not be optimal in children with chronic kidney diseases, they should still be vaccinated according to the schedule as much as possible before transplantation (44-46). Live virus vaccines which include measles, mumps, rubella and varicella are not safe to be administered after transplantation. Therefore, these vaccines should be given while the children are still waiting for the transplantation. Often when transplantations occur in very young children, the immunization schedule may have to be delayed until after the transplantation (43). According to the AST guidelines, most transplant centers resume vaccination with inactivated vaccines at approximately three to six months after transplantation when the dosages of the immunosuppressants are usually lower (43). Table 2 depicts the vaccines that are considered to be safe after renal transplantation. Studies from children and adults allograft recipients suggest that booster doses of some vaccines are often required to maintain adequate protections, albeit guidelines for antibody monitoring post transplantation have not been established (46-48).
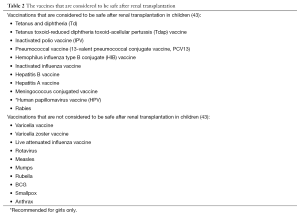
Full table
Special considerations of vaccinations in allograft
recipients
Although most children would have been vaccinated against varicella prior to transplantation, some patients remain non-immune and may require re-vaccination. Despite some anecdotal data suggesting the efficacy in immunizing children long after transplantation, current literature is not sufficient to support the safety of varicella vaccine in all patients (49-51). Allograft recipients are also prone to have zoster reactivation, but the varicella zoster (Zostavax) vaccine, which has been approved to be used in immunocompetent adults, has not been proven to be safe in immunosuppressed children (52). There are also insufficient data to confirm the efficacy and safety of rotavirus vaccine in children after renal transplantation (43).
Special considerations of vaccinations to family members of allograft recipients
In general, siblings and close contacts of allograft recipients should receive vaccination according to current recommendations, including yearly influenza vaccines (43). The only exception is oral poliovirus vaccine as the virus may be transmissible to the transplant recipient (43).
Varicella vaccines
There are conflicting opinions on the safety of administration of varicella vaccine in household contacts of transplant recipients. Although both American Academy of Pediatrics (AAP) and AST support the use of the varicella vaccine in household contacts, both organizations recommend isolation if rashes develop and they should not be in contact of the transplant recipients (53,54). If rashes develop in the transplant recipient, the transplant center should be notified and anti-viral therapy should be administered immediately.
Rotavirus vaccine
There is no guideline at the present moment on whether young siblings of transplant recipients should be immunized against rotavirus. Although it has been documented in siblings of transplant recipient that rotavirus shedding happened after the first vaccination, most experts consider the risk of household transmission is less severe than the wild-type rotavirus. Physicians should weigh the potential risks against the benefits in recommending vaccination to household members of transplant recipients. Nonetheless, caregivers to allograft recipients should always observe good hand washings hygiene especially after changing diapers or handling fecal matter of the vaccinated children in order to minimize household transmission (48).
Cardiovascular and metabolic complications
Chronic kidney disease is a well established and independent risk factor for cardiovascular morbidity (55). Children on dialysis have significantly higher risk of early death due to cardiovascular complications and the risks are not reversed after transplantation (56,57). In addition, most of the available immunosuppressive drugs at the present moment, especially the corticosteroids, tacrolimus and sirolimus, can contribute to hypertension, hyperlipidemia, diabetes and obesity, thus continuing to increase the risk of cardiovascular complications in children after renal transplantation (14,58-62). The significant roles of pediatricians to modify these risk factors cannot be overemphasized (63,64).
Children with dyslipidemia and diabetes secondary to immunosuppressive drugs are initially managed with adjustment of the dose or conversion to other drugs. Pharmacological treatments may become necessary but dietary and lifestyle modifications should always be part of the plan. Although the nephrology teams manage most of these metabolic derangements, the adjunctive roles of pediatricians cannot be overemphasized. As most families have had long-term relationships with their pediatricians prior to transplantation, they are in a much better position to encourage lifestyle modification and help the transplant team to monitor the child’s progress.
Gastrointestinal issues
The most common gastrointestinal complaints in children after renal transplantation are abdominal pain and diarrhea. Although severe illness such as esophageal necrosis and pancreatitis have been reported, peptic ulcer has been by far the most frequent cause of acute abdominal pain (65,66). However, ulcer diseases secondary to corticosteroids are becoming less frequently seen with the more rapid tapering regimen and astute use of ulcer protective agents. Health care providers need to consider other etiologies such as CMV colitis if the pain is associated with diarrhea and resistant to antacids.
Diarrhea is becoming more prevalent in children after renal transplantation. CMV, rotavirus and other pathogens are able to cause severe diarrhea in immunosuppressed children. The frequency of diarrhoea has been noticed to be higher in patients receiving mycophenolate mofetil as part of their anti-rejection regimen, which may be due to the inhibition of crypt cells divisions in colon (67,68). Physicians need to differentiate whether the underlying cause is infectious or non-infectious in origin. As mycophenolate may worsen the diarrhea, the dose may be required to be reduced during the acute phase. Fluid management is also important during this phase. Studies have also showed that tacrolimus levels tend to be elevated during diarrhoea and subsequently need to be monitored closely (69). If in doubt, or if the doses of immnosuppressive therapies require adjustments, the pediatrician should consult with the transplant team and the child may need to be seen earlier by the team.
Nutritional issues
While most children do not have difficulty in transitioning from the previously restrictive renal diet to the normal regular diet after transplantation, pediatricians and family physicians may need to be aware of the factors that may potentially impede this transition. For example, some children depend on tube feeding before the transplantation and may display oral motor dysfunction. Anorexia is commonly seen in children with chronic illness and it may become challenging to introduce new food items and increase caloric intake. In contrast to this, some children may need to be continued on restrictive diet due to hyperlipidemia and hyperglycemia. Pediatricians play essential roles in managing the nutritional needs in children after renal transplantation. Regular documentation of growth parameters on appropriate growth curves can aid the nephrology team to evaluate their growth after transplantation, so interventions such as nutritional supplementation and growth hormone can be implemented promptly if indicated.
Psychosocial development
The process of renal transplants has been reported as stressful by adolescent patients, with stress impacting on: perceptions of body image, desire for normalcy, physical pain and difficulties with communication (70). An understanding of the stressors adolescent experience post-renal transplant is essential for preventing and advocating for mental health and psychosocial development of these at-risk adolescents. Adolescents have reported the need for assisting with self-management, meaningful social support, academic transitions and family relations.
Quality of life
As long-term survival from renal transplantation is as well established outcome, an increasing number of children and families are now living longer healthier lives. However, the restoration of renal function does not necessarily translate to a normal life. The focus on assessment on healthy organ function is important, but the evaluation of a child and family's quality of life has also be identified as essential (71). Pediatricians are encouraged to assist children and their families by assessing quality of life post-renal transplant. Children with renal transplants when compared to health reference scores should greater concern with physical appearance, physical symptoms, and increased difficulty with peer and family relations and school disruption (71). The stress pre and post renal transplant is not only isolated to children. Parents reports on quality of life tools has shown notable differences between results for children and parents, with parents having low scores on parental emotional function suggesting the negative impact of renal transplantation on family function (71). Pediatricians are in a position to recognize and assist families and children post-renal transplantation by screening for stressors, assessing quality of life and referring families and children to appropriate counseling services.
Non-adherence
Non-adherence in the pediatric transplant population, especially in adolescents, is well documented and has led to significant morbidity including graft rejections (72,73). Pediatricians play supportive roles by encouraging adolescents and monitoring for adherence. The side effects of the medications on the body image of adolescents are well documented. Feelings of vulnerability may evolve into risk taking behaviors and non-adherence (74). Anxiety has also been identified as a significant factor associated with medication adherence (75). While it is crucial to be sensitive to the children’s concerns and stresses, the consequences of not taking immunosuppressive medications should be addressed on a regularly base and be reinforced repeatedly during routine checkups. When intervention is needed, the pediatrician can work with the nephrology team to arrange counseling and psychosocial support.
Other cosmetic issues
Gingival hyperplasia is a common side effect in patients receiving cyclosporine. Although cyclosporine has been replaced by tacrolimus as the mainstay of immunosuppressive therapy, some children who received their allograft relatively long time ago might still be on this medication. Good oral hygiene with brushing, flossing and plaque control may improve the gum conditions but these interventions may not avert gingival hyperplasia. Gum hypertrophy may recede over time but some children may require gingivectomy. Antibiotic prophylaxis for endocarditis should be considered before these dental procedures (76).
Daily activities issues
Children usually become more active and energetic after renal transplantation, and they should be encouraged to participate in age appropriate activities as soon as tolerated. At our center, children may resume all activities after 8 weeks of transplantation but are usually advised to avoid heavy lifting for 6 months. Although not encouraged, if the children prefer to participate in vigorous contact sports such as football and wrestling, adequate and appropriate protective measures need to be reviewed.
Most children are ready to return to school within 3 months, a few hours each day until they can tolerate a full day. It is not uncommon to come across parents who are overprotective and hesitate to allow their children to return school because of the fear of exposure to infections. The pediatrician is pivotal in counseling these parents by reiterating the benefits of allowing their children to return to school and thus interacting with their peers. Pediatricians can act as advocates for children and liaise between the school and health care team. If children are well enough, collaboration with the school while children are at home, may allow for tutoring in the home environment by school teachers, so as to alleviate stress for the child when they do return to school. Programs in the UK demonstrating an alliance between hospital care providers and educators has proven effective (77). The primary care physician may also facilitate the transition by coordinating with the school nurses to ensure that the child will receive his anti-rejection medications on time.
Conclusions
The growing population of kidney transplant recipients and their improved long term survival rates will continue to increase the need for long term care by their primary care physicians. The transplant team does not supplant the pediatricians who remain the providers for their health maintenance needs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Horslen S, Barr ML, Christensen LL, et al. Pediatric transplantation in the United States, 1996-2005. Am J Transplant 2007;7:1339-58. [PubMed]
- Transplants in the U.S. by Recipient Age. 2011 cited; Available online: http://optn.transplant.hrsa.gov/latestData/rptData.asp
- Tejani A, Sullivan EK, Fine RN, et al. Steady improvement in renal allograft survival among North American children: a five year appraisal by the North American Pediatric Renal Transplant Cooperative Study. Kidney Int 1995;48:551-3. [PubMed]
- Gulati A, Sarwal MM. Pediatric renal transplantation: an overview and update. Curr Opin Pediatr 2010;22:189-96. [PubMed]
- Webster A, Woodroffe RC, Taylor RS, et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005.CD003961. [PubMed]
- Harmon W, Meyers K, Ingelfinger J, et al. Safety and efficacy of a calcineurin inhibitor avoidance regimen in pediatric renal transplantation. J Am Soc Nephrol 2006;17:1735-45. [PubMed]
- Knight RJ, Villa M, Laskey R, et al. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant 2007;21:460-5. [PubMed]
- Zuber J, Anglicheau D, Elie C, et al. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant 2008;8:1471-9. [PubMed]
- NAPRTCS 2011 Annual Transplant Report.
- Lau KK, Haddad MN, Berg GM, et al. Rapid steroid discontinuation for pediatric renal transplantation: a single center experience. Pediatr Transplant 2007;11:504-10. [PubMed]
- Lau KK, Berg GM, Schjoneman YG, et al. Extended experience with a steroid minimization immunosuppression protocol in pediatric renal transplant recipients. Pediatr Transplant 2010;14:488-95. [PubMed]
- Höcker B, John U, Plank C, et al. Successful withdrawal of steroids in pediatric renal transplant recipients receiving cyclosporine A and mycophenolate mofetil treatment: results after four years. Transplantation 2004;78:228-34. [PubMed]
- Sarwal MM, Vidhun JR, Alexander SR, et al. Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation 2003;76:1331-9. [PubMed]
- Lau KK, Tancredi DJ, Perez RV, et al. Unusual pattern of dyslipidemia in children receiving steroid minimization immunosuppression after renal transplantation. Clin J Am Soc Nephrol 2010;5:1506-12. [PubMed]
- Chan K, Taube D, Roufosse C, et al. Kidney transplantation with minimized maintenance: alemtuzumab induction with tacrolimus monotherapy-an open label, randomized trial. Transplantation 2011;92:774-80. [PubMed]
- Christians U, Jacobsen W, Benet LZ, et al. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet 2002;41:813-51. [PubMed]
- Peynaud D, Charpiat B, Vial T, et al. Tacrolimus severe overdosage after intake of masked grapefruit in orange marmalade. Eur J Clin Pharmacol 2007;63:721-2. [PubMed]
- Gaston RS. Maintenance immunosuppression in the renal transplant recipient: an overview. Am J Kidney Dis 2001;38:S25-35. [PubMed]
- Peterson PK, Balfour HH Jr, Fryd DS, et al. Fever in renal transplant recipients: causes, prognostic significance and changing patterns at the University of Minnesota Hospital. Am J Med 1981;71:345-51. [PubMed]
- Snyder JJ, Israni AK, Peng Y, et al. Rates of first infection following kidney transplant in the United States. Kidney Int 2009;75:317-26. [PubMed]
- Knoll G. Trends in kidney transplantation over the past decade. Drugs 2008;68:3-10. [PubMed]
- NAPRTCS 2010 Annual Report; Available online: https://web.emmes.com/study/ped/annlrept/annlrept.html
- Dantas SR, Kuboyama RH, Mazzali M, et al. Nosocomial infections in renal transplant patients: risk factors and treatment implications associated with urinary tract and surgical site infections. J Hosp Infect 2006;63:117-23. [PubMed]
- Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant 2006;20:401-9. [PubMed]
- Chavers BM, Solid CA, Gilbertson DT, et al. Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol 2007;18:952-9. [PubMed]
- Valera B, Gentil MA, Cabello V, et al. Epidemiology of urinary infections in renal transplant recipients. Transplant Proc 2006;38:2414-5. [PubMed]
- Lyerová L, Viklický O, Nemcová D, et al. The incidence of infectious diseases after renal transplantation: a single-centre experience. Int J Antimicrob Agents 2008;31:S58-62. [PubMed]
- Silva A, Rodig N, Passerotti CP, et al. Risk factors for urinary tract infection after renal transplantation and its impact on graft function in children and young adults. J Urol 2010;184:1462-7. [PubMed]
- Rivera-Sanchez R, Delgado-Ochoa D, Flores-Paz RR, et al. Prospective study of urinary tract infection surveillance after kidney transplantation. BMC Infect Dis 2010;10:245. [PubMed]
- John U, Everding AS, Kuwertz-Bröking E, et al. High prevalence of febrile urinary tract infections after paediatric renal transplantation. Nephrol Dial Transplant 2006;21:3269-74. [PubMed]
- Goto N, Oka S. Pneumocystis jirovecii pneumonia in kidney transplantation. Transpl Infect Dis 2011;13:551-8. [PubMed]
- Kranz B, Vester U, Wingen AM, et al. Acute rejection episodes in pediatric renal transplant recipients with cytomegalovirus infection. Pediatr Transplant 2008;12:474-8. [PubMed]
- De Keyzer K, Van Laecke S, Peeters P, et al. Human cytomegalovirus and kidney transplantation: a clinician’s update. Am J Kidney Dis 2011;58:118-26. [PubMed]
- Kremers WK, Devarbhavi HC, Wiesner RH, et al. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant 2006;6:1017-24. [PubMed]
- Rostaing L, Wéclawiak H, Mengelle C, et al. Viral infections after kidney transplantation. Minerva Urol Nefrol 2011;63:59-71. [PubMed]
- Ryu HJ, Hahn JS, Kim YS, et al. Complete resolution of posttransplant lymphoproliferative disorder (diffuse large B-cell lymphoma) with reduction of immunosuppressive therapy. Yonsei Med J 2004;45:527-32. [PubMed]
- Pollara CP, Corbellini S, Chiappini S, et al. Quantitative viral load measurement for BKV infection in renal transplant recipients as a predictive tool for BKVAN. New Microbiol 2011;34:165-71. [PubMed]
- Boeckh M. Prevention of VZV infection in immunosuppressed patients using antiviral agents. Herpes 2006;13:60-5. [PubMed]
- Weinstock DM, Boeckh M, Boulad F, et al. Postexposure prophylaxis against varicella-zoster virus infection among recipients of hematopoietic stem cell transplant: unresolved issues. Infect Control Hosp Epidemiol 2004;25:603-8. [PubMed]
- Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med 2010;363:1451-62. [PubMed]
- Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med 2003;349:2326-33. [PubMed]
- Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet 2011;378:1428-37. [PubMed]
- Danzinger-Isakov L, Kumar D, AST Infectious Diseases Community of Practice. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant 2009;9:S258-62. [PubMed]
- Hibberd PL, Rubin RH. Approach to immunization in the immunosuppressed host. Infect Dis Clin North Am 1990;4:123-42. [PubMed]
- Molrine DC, Hibberd PL. Vaccines for transplant recipients. Infect Dis Clin North Am 2001;15:273-305. xii. [PubMed]
- Gangappa S, Kokko KE, Carlson LM, et al. Immune responsiveness and protective immunity after transplantation. Transpl Int 2008;21:293-303. [PubMed]
- Diana A, Posfay-Barbe KM, Belli DC, et al. Vaccine-induced immunity in children after orthotopic liver transplantation: a 12-yr review of the Swiss national reference center. Pediatr Transplant 2007;11:31-7. [PubMed]
- Posfay-Barbe KM, Siegrist CA. Immunization and transplantation--what is new and what is coming? Pediatr Transplant 2009;13:404-10. [PubMed]
- Weinberg A, Horslen SP, Kaufman SS, et al. Safety and immunogenicity of varicella-zoster virus vaccine in pediatric liver and intestine transplant recipients. Am J Transplant 2006;6:565-8. [PubMed]
- Kraft JN, Shaw JC. Varicella infection caused by Oka strain vaccine in a heart transplant recipient. Arch Dermatol 2006;142:943-5. [PubMed]
- Levitsky J, Te HS, Faust TW, et al. Varicella infection following varicella vaccination in a liver transplant recipient. Am J Transplant 2002;2:880-2. [PubMed]
- Safety and Immunogenicity of Zostavax Vaccine in Patients Undergoing Living Donor Kidney Transplantation. Available online: http://clinicaltrials.gov/ct2/show/NCT00940940
- Varicella-zoster infections. 28 ed. Elk Grove Village, IL: American Academy of Pediatrics, 2009.
- Pergam SA, Limaye AP. AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant 2009;9:S108-15. [PubMed]
- Sciarretta S, Valenti V, Tocci G, et al. Association of renal damage with cardiovascular diseases is independent of individual cardiovascular risk profile in hypertension: data from the Italy - Developing Education and awareness on MicroAlbuminuria in patients with hypertensive Disease study. J Hypertens 2010;28:251-8. [PubMed]
- Levey AS, Beto JA, Coronado BE, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 1998;32:853-906. [PubMed]
- Querfeld U. The clinical significance of vascular calcification in young patients with end-stage renal disease. Pediatr Nephrol 2004;19:478-84. [PubMed]
- Chueh SC, Kahan BD. Dyslipidemia in renal transplant recipients treated with a sirolimus and cyclosporine-based immunosuppressive regimen: incidence, risk factors, progression, and prognosis. Transplantation 2003;76:375-82. [PubMed]
- Kasiske BL. Risk factors for accelerated atherosclerosis in renal transplant recipients. Am J Med 1988;84:985-92. [PubMed]
- Fellström B. Risk factors for and management of post-transplantation cardiovascular disease. BioDrugs 2001;15:261-78. [PubMed]
- Morales JM, Dominguez-Gil B. Cardiovascular risk profile with the new immunosuppressive combinations after renal transplantation. J Hypertens 2005;23:1609-16. [PubMed]
- Morales JM. Cardiovascular risk profile in patients treated with sirolimus after renal transplantation. Kidney Int Suppl 2005.S69-73. [PubMed]
- Hanevold CD, Ho PL, Talley L, et al. Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 2005;115:352-6. [PubMed]
- Silverstein DM. Risk factors for cardiovascular disease in pediatric renal transplant recipients. Pediatr Transplant 2004;8:386-93. [PubMed]
- Trappe R, Pohl H, Forberger A, et al. Acute esophageal necrosis (black esophagus) in the renal transplant recipient: manifestation of primary cytomegalovirus infection. Transpl Infect Dis 2007;9:42-5. [PubMed]
- Fernández-Cruz L, Targarona EM, Cugat E, et al. Acute pancreatitis after renal transplantation. Br J Surg 1989;76:1132-5. [PubMed]
- Phatak UP, Seo-Mayer P, Jain D, et al. Mycophenolate mofetil-induced colitis in children. J Clin Gastroenterol 2009;43:967-9. [PubMed]
- Helderman JH, Goral S. Gastrointestinal complications of transplant immunosuppression. J Am Soc Nephrol 2002;13:277-87. [PubMed]
- Frühwirth M, Fischer H, Simma B, et al. Elevated tacrolimus trough levels in association with mycophenolate mofetil-induced diarrhea: a case report. Pediatr Transplant 2001;5:132-4. [PubMed]
- Korus M, Stinson JN, Pool R, et al. Exploring the information needs of adolescents and their parents throughout the kidney transplant continuum. Prog Transplant 2011;21:53-60. [PubMed]
- Anthony SJ, Pollock Barziv S, Ng VL. Quality of life after pediatric solid organ transplantation. Pediatr Clin North Am 2010;57:559-74. [PubMed]
- Feinstein S, Keich R, Becker-Cohen R, et al. Is noncompliance among adolescent renal transplant recipients inevitable? Pediatrics 2005;115:969-73. [PubMed]
- Rianthavorn P, Ettenger RB, Malekzadeh M, et al. Noncompliance with immunosuppressive medications in pediatric and adolescent patients receiving solid-organ transplants. Transplantation 2004;77:778-82. [PubMed]
- Bollini P, Tibaldi G, Testa C, et al. Understanding treatment adherence in affective disorders: a qualitative study. J Psychiatr Ment Health Nurs 2004;11:668-74. [PubMed]
- Wu YP, Aylward BS, Steele RG. Associations between internalizing symptoms and trajectories of medication adherence among pediatric renal and liver transplant recipients. J Pediatr Psychol 2010;35:1016-27. [PubMed]
- Goldman KE. Dental management of patients with bone marrow and solid organ transplantation. Dent Clin North Am 2006;50:659-76. viii. [PubMed]
- Poursanidou K, Garner P, Watson A. Hospital--school liaison: perspectives of health and education professionals supporting children with renal transplants. J Child Health Care 2008;12:253-67. [PubMed]


