
Semiquantitative analysis of power doppler ultrasonography versus Tc-99m DMSA scintigraphy in diagnostic and severity assessment of acute childhood pyelonephritis
Introduction
Febrile urinary tract infection (UTI) is the second most common infectious disease in infants and children and the most common disease in children under the age of two (1). About 50–90% children with febrile UTI have renal parenchyma involvement which indicates acute pyelonephritis (APN). Without prompt diagnosis and treatment, 40–60% of children with this condition will develop permanent renal scarring with sequelae of hypertension and renal failure (2). The diagnosis of APN is initially made through urinalysis and the presence of clinical symptoms, which makes it hard to differentiate it from lower UTI (3). Therefore, a fast and accurate diagnosis is required.
According to the literature, Tc-99m dimercaptosuccinic acid (DMSA) renal scintigraphy is the most reliable diagnostic tool for suspected APN, and it is able to evaluate the degree of renal parenchymal involvement (4-6). However, DMSA scintigraphy has the disadvantages of ionizing radiation exposure, invasiveness, and relatively high cost, rendering it unappealing to children and parents (7). The recent development of power Doppler ultrasonography (PDU) shows encouraging results in diagnosing APN, with a reported accuracy of 89% (7-10). With additional advantages of being radiation-free, convenient, and low cost, PDU may be a new tool in APN diagnosis (7-10). However, experiments in clinic and on piglet models revealed the lower accuracy of PDU compared with DMSA scintigraphy (11-13).
Thus, the purpose of this study was to assess the diagnostic and predictive value of PDU for APN in children, with the help of a new semiquantitative system, and to determine whether PDU may supersede Tc-99m DMSA in renal scintigraphy.
We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/tp-20-59).
Methods
Clinical and biologic findings
From December 2016 to April 2017, we evaluated 92 infants and children (36 girls and 56 boys; aged 36 days to 10 years; mean age 18.0±2.8 months) who were admitted to the Pediatric Nephrology Department with potential APN. The retrospective trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional board of The Second Affiliated Hospital of Wenzhou Medical University (No. L-2019-08), and informed consent was given by all the patients and/or their guardians. The inclusion criteria were as follows: (I) age between 1 week and 16 years (inclusive); (II) clinical diagnosis of APN including (i) fever >38.5 °C or localized symptoms of APN, (ii) pyuria or a positive urine culture (>10 white blood cells per cubic millimeter and bacteriuria to the extent of 104 colony-forming units per milliliter) (14,15); (III) children and parents’ consent to accept both PDU and Tc-99m DMSA renal scintigraphy. Exclusion criteria included several previous episodes of APN and congenital structural anomalies of the urinary system. All children underwent a standardized clinical examination including abdominal and lumbar fossa palpation, temperature and blood pressure measurement, standardized blood studies including C-reactive protein (CRP), differential blood count, iconography, renal function, and liver function analysis. A midstream urine sample was taken for N-acetylglucosaminidase (NAG), β2-microglobulin (β2-MG), dipstick analysis, and culturing. Clinical examination results, urine samples and blood samples were collected on admission before commencing usual intravenous antibiotic treatment, and PDU and Tc-99m DMSA renal scintigraphy were performed within 72 hours of admission. Young, uncooperative patients were duly sedated before examination.
DMSA
Tc-99m DMSA renal scintigraphy was performed using the standard protocol (16). In brief, a dose of 3.7 MBq/kg (0.1 mCi/kg) Tc-99m DMSA was intravenously injected, and 2–4 h later, images were obtained in the planar anterior, posterior, and right and left posterior oblique using an Orbiter Siemens gamma camera with a low-energy high-resolution parallel-hole collimator. Images were obtained for 300,000–500,000 counts on a 256×256 matrix format. International Radionuclide Nephrourology Group (IRN) consensus criteria were used for interpretation of DMSA scintigraphy results. To standardize the interpretation, an empiric 9-point semiquantitative analysis that evaluated the lesion size and radioactivity of each kidney was performed. The kidneys were divided into three zones (the upper pole, midzone, and lower pole), and the radioactive uptake of each zone was scored from 0 (no uptake) to 3 (normal uptake). The sum of the zone scores was considered the total score of each kidney. An 8–9 score was considered grade 0 (normal), a 6–7 score was considered grade I (mildly abnormal), a 4–5 score was considered grade II (moderately abnormal), and a 0–3 score was considered grade III (severely abnormal) (17,18).
Two expert senior physicians who were blinded to the other examination results and clinical information analyzed all the images. If disagreements occurred, a final diagnosis was made after discussion.
PDU
Grey-scale ultrasonography and PDU were performed using the Esaote MyLab Class C with variable-frequency (2.5–4.0 MHz) curved transducers in prone and supine positions for both axial and longitudinal scans. Grey-scale ultrasonography of the kidneys was performed to exclude structural urinary tract anomalies and to evaluate size, echogenicity, and stasis. The parameters of PDU were individualized for each kidney in every patient for the optimized visualization of the parenchymal perfusion map. To standardize the interpretation, the same 9-point semiquantitative analysis was performed. Briefly, each kidney was divided into three zones, and the perfusion of each zone was scored from 0 (no perfusion) to 3 (normal perfusion). A grade 0, I, II, or III was determined by a total score of 8–9, 6–7, 4–5, and 0–3 in each kidney, respectively. Grade 0 indicated a normal kidney, and grades I–III indicated the respective degrees of abnormality.
Two expert senior physicians who were blinded to the results of the other imaging examinations and clinical information analyzed all the images. If disagreements occurred, a final diagnosis was made after discussion. To compare the scintigraphic and sonographic images, the grades of each kidney were evaluated.
Statistics
Statistical analysis was performed on all 92 children comprising 184 kidneys. Inter-rater agreement between PDU and DMSA scintigraphy was assessed by calculation of the kappa coefficient (Ko) and with the χ2 test using SPSS 19.0 software. Based on Ko values, the strength of agreement was classified as very good (81–100%), good (61–80%), moderate (41–60%), fair (21–40%), and poor (<20%) (8). The diagnostic values (sensitivity, specificity, predictive values, and accuracy) of PDU were assessed with contingency tables. A P value ≤0.05 was considered significant.
Results
Comparison of characteristics and biologic findings in children
All 92 children met the diagnostic criteria of febrile APN. Among them, 52 (56.5%) were abnormal (grade I–III) on DMSA scintigraphy, and 58 (63.0%) were abnormal on PDU. Based on urine analysis, 83 patients (90.2%) had increased neutrophil esterase in urine, 30 patients (32.6%) had positive urinary nitrite, 24 patients (26.1%) had elevated urine NAG, and 19 patients (20.7%) had increased urine β2-MG. Blood analysis revealed high CRP (≥20 mg/L) and elevated white blood cell count in 76 (82.6%) and 79 patients (85.9%) respectively. None of the patients had abnormal renal function, albumin, or blood electrolytes. Urinary grey-scale ultrasonography revealed renal cortical echo changes in 5 patients (5.4%), kidney enlargement in 33 patients (35.9%), and kidney atrophy in 2 patients (2.2%). The positive rate of grey-scale ultrasonography was 40.2%.
Semiquantitative analysis of PDU and DMSA and their comparison
PDU and DMSA scintigraphy were performed successfully on all 184 kidneys. In all, 68 kidneys appeared abnormal on DMSA scintigraphy, of which 61 showed the same on PDU (Table 1, Figures 1,2). Meanwhile, 116 kidneys presented normal (grade 0) on DMSA scintigraphy, and 93 of them appeared the same on PDU. Thus, PDU showed a sensitivity of 89.7%, a specificity of 80.2%, and an accuracy of 83.7% (P<0.05). Positive and negative predictive values of PDU were 72.6% and 93.0% respectively (P<0.05).

Full table
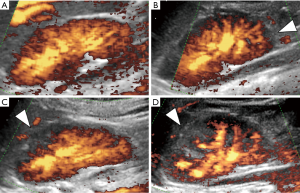
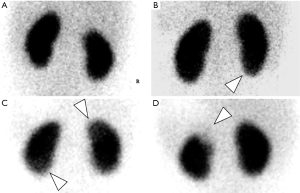
To determine whether the grade detected by PDU accorded with DMSA scintigraphy, we ran a Ko analysis which uncovered a moderate strength of agreement (41%, P<0.05).
Semiquantitative analysis of PDU and DMSA with different age
Children were divided into two groups: under 6 months (inclusive, group 1) and over 6 months (group 2), with 102 and 82 kidneys in each group respectively (Table 2). In group 1, 58 kidneys appeared normal on both PDU and DMSA scintigraphy while 27 kidneys appeared abnormal. In group 2, 35 kidneys revealed normal while 34 revealed abnormal on both DMSA scan and PDU detection. Thus, the sensitivity, specificity, accuracy, and positive and negative predictive values were 87.1%, 81.7%, 83.3%, 67.5%, and 93.5% for group 1 (P<0.05), and 91.9%, 77.8%, 84.1%, 77.3%, and 92.1% for group 2 (P<0.05).

Full table
To determine whether the consistency of grade was related to age, Ko analysis was conducted, with the results showing a fair strength of agreement between PDU and DMSA scintigraphy for group 1 (38%, P<0.05) and a moderate one for group 2 (43%, P<0.05).
Discussion
APN is an infection involving the renal parenchyma and is considered to be one of the most serious bacterial illnesses of childhood. About 40–60% of APN children will develop renal scarring and a possible long-term morbidity of hypertension and renal failure (2,3). The clinical diagnosis of APN is mainly based on symptoms, signs, and laboratory results. However, the symptoms are often vague and influenced by the child’s age, inflammatory immune response, and the virulence of the organism (3). In very young infants (<2–3 months), there can be non-specific symptoms (slow weight gain, drowsiness etc.) which further confound diagnosis. Children with ambiguous symptoms have been reported to be at higher risk of complications, such as sepsis and meningitis (3). These facts emphasize the importance of rapid and accurate diagnosis of APN.
Blood and urine samples are collected when UTI is suspected. The literature shows that the elevation of CRP levels and white blood cell count can imply the presence of renal involvement, but their sensitivity and specificity are vague (3,19). Positive urinary leukocyte esterase and nitrite result from the presence of white blood cells in urine and bacteriuria and may be related to UTI. Their combination may even be more helpful in diagnosing APN than when each is used alone, but their ability to diagnose bacteriuria is significantly less reliable in younger children (20-22). Renal damage may cause NAG and β2-MG excretion in urine (17,23). Studies show that detecting these factors can enable the early diagnosis of APN in children (23). However, urine NAG excretion normalizes rapidly during APN, resulting in low sensitivity (17,24), while urinary β2-MG often tests normal in the earlier stages of renal injury (23). In accordance with the relevant literature, our data confirmed a positive rate of 82.6% in CRP, 85.9% in white blood cell count, 90.2% in urine leukocyte esterase, 32.6% in urine nitrite, 26.1% in urine NAG, and 20.7% urine β2-MG. With the abnormal rate of 63.0% on DMSA scintigraphy in our study, those examinations can rarely permit a diagnostic value for APN.
Changes in APN kidneys appeared on gray-scale ultrasonic imaging, and included increased renal size, mass, and triangular or renal sinus hyperechogenicity (15). These features are related to renal swelling and loss of corticomedullary differentiation (8). In recent studies using DMSA scintigraphy as the gold standard, the sensitivity of grey-scale ultrasonography was shown to be inferior to that of DMSA and Doppler ultrasonography for the diagnosis of APN (15,25). Mitra’s study revealed a positive rate of 40% for patients on grey scale ultrasonography, which was lower than that on DMSA (56%) and PDU (50%) (7). In our study, we found cortical echo changes in 5.4% of patients, renomegaly in 35.9%, and kidney atrophy in 2.2%. A positive rate of 40.2% presents a lower diagnostic value of grey-scale ultrasonography than DMSA (56.5%) and PDU (63.0%).
Many imaging techniques have been compared for their ability to diagnose APN in the pediatric population. Their theoretical basis mainly relies on observation of reduced renal blood flow, which is attributed to intravascular granulocyte aggregation, edema, and dysregulated transport to the intratubular neutrophils. In sequelae, arteriolar or capillary occlusion, toxic enzymes, or superoxide production and accumulation occur (26). Tc-99m DMSA renal scintigraphy is considered the gold standard for the confirmation of APN (14,27) with a 91% and 99% sensitivity and specificity, having been reported, respectively (4). The invasiveness of the procedure, ionizing radiation exposure, and cost limit its appeal to children and parents. Meanwhile, normal DMSA findings cannot fully exclude APN (10), and its false-positive findings can lead to the misdiagnosis of APN. Furthermore, the results from DMSA scintigraphy are hard to quantify, and the photopenic lesion area or change in radioactivity cannot be accurately assessed. Researchers have exhaustively attempted to resolve this problem: Chiou introduced an empiric-threshold method for volume determination while Linne and Hitzel conducted score systems to measure the volume of an APN focus and calculate the degree of DMSA uptake defect (17,18). These attempts were unsuccessful, and a new imaging method is urgently required.
PDU is a relatively new, non-invasive technique for renal vascular visualization that does not involve exposure to radiation. Mixed results have emerged across studies, while here, a promising diagnostic value was noted due to high sensitivity (89%) and specificity (92.3%) (7,9). Data from 57 children with a mean age of 5 years showed a sensitivity of 80% and a specificity of 81% (15). However, lower sensitivity was found in Bukov’s (74%) and Stogianni’s (73.8%) data (8,10). Experiments using a pig model also demonstrated a low sensitivity (56.6%) of PDU compared with DMSA scintigraphy (92.1% sensitivity) (11,12). We made a seriously study of them, and found the PDU results had been calculated qualitatively, which may have led to its determination of low sensitivity and specificity (8,10-12). Despite this, PDU has shown some success in revealing lesions that remained invisible on DMSA scintigraphy (10), which may give credibility to its diagnostic value. Thus, we synthesized the scoring systems of DMSA scintigraphy (17,18), and were first to introduce a 9-point semiquantitative analysis system in PDU in order to produce a more reliable and accurate diagnostic result. Meanwhile, we classified kidneys into four grades to assess the severity of APN and predict its development. The diagnostic ability and consistency of both methods were compared to discern whether PDU could replace DMSA scintigraphy.
With the new semiquantitative analysis system, our data confirmed a high sensitivity (89.7%) and specificity (80.2%) of PDU which correlated well with the results of other experimental research (7,9,10). We had only 7 (of 184) false-negative findings (4 in group 1 and 3 in group 2), all from the upper and lower pole of the kidney. This may be explained by the partial venous congestion caused by edema, difficulty in differentiating normal from increased flow, and the absence of a hepatic acoustic window in the upper pole of the left kidney (7,10). Furthermore, the normal heterogeneity of DMSA uptake within the renal cortex might mimic areas of abnormally decreased uptake (10). The 23 false-positive results (13 in group 1 and 10 in group 2) might be explained by technical artifacts, rib artifacts, respiratory motion, or intestinal gas (10). However, with the P value below 0.05, we could not verify whether PDU could supersede DMSA scintigraphy in diagnosing APN.
To further assess the value of PDU for detecting APN and to clarify the consistency between the two methods, grouping and Ko analysis were performed. We divided children into two groups: younger than 6 months (inclusive, group 1) and older than 6 months (group 2). The sensitivity and specificity for group 1 were 87.1% and 81.7% (P<0.05), respectively, and 91.9% and 77.8% for group 2, respectively (P<0.05), indicating that the diagnostic value of PDU increased with age. This might be due to age-related kidney development and a different pathophysiology in those younger than 6 months (3,15). Furthermore, the more pronounced respiratory motion of younger children might have compromised the reliability of PDU in APN diagnosis. A moderate level of agreement (41%, P<0.05) between PDU and DMSA scintigraphy in distinguishing the grade was observed in our study, and fair and moderate agreement was found for group 1 (38%, P<0.05) and group 2 (43%, P<0.05), respectively. These results agreed with our data above. Thus, we can conclude that PDU has the potential to obviate DMSA scintigraphy in children older than 6 months for APN diagnosis.
With the result that lesions detected by ultrasonography might remain invisible on DMSA scintigraphy, we found that the severity detected with PDU was consistent with that of DMSA scintigraphy to some degree (15). Published studies have shown that abnormal findings of DMSA scintigraphy regarding APN in children indicated a high risk of renal scarring, and renal scarring was a precipitator of decline in renal function (1,15). Besides this, the extent of DMSA uptake defect was found to correlate with clinical significance and the need for continuous prophylaxis (10,17). Indeed, if treatment is not started within the first week, there would be no difference in the prognosis (27). Roupakias and Yuan-Yow’s study uncovered a correlation between the likelihood of renal scarring and the size and area of renal parenchymal involvement (1,27). Since the grade in our research depended mainly on hypoperfusion or defect area and severity, we can deduce that the PDU grade can predict the prospect of the development of APN, especially for children older than 6 months.
Our study had some limitations: (I) we used a relatively small sample size combined with a wide age range (36 days to 10 years), which might have introduced sample selection bias; (II) there were no pathological results for each kidney, and since imaging technology was subjective, the diagnostic bias could not be avoided; (III) we did not re-examine patients 6 or 12 months after their discharge to assess the relationship between PDU grade and prognosis. Therefore, adequate follow-up, including PDU and DMSA scintigraphy to assess renal scarring, should be considered for future studies.
Conclusions
With the help of a semiquantitative analysis system, we can conclude that PDU presents a promising alternative for diagnosing APN in children, especially those older than 6 months. However, because of the false-positive results and moderate agreement, PDU may over diagnose or misjudge the development of APN, to an extent. The predictive value of PDU should increase with the technical advancement of the equipment and the accumulation of radiologist experience.
Acknowledgments
Funding: This study was supported by the Natural Science Foundation of Zhejiang Province (LSY19H180008).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-59
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-59
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-59). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional board of The Second Affiliated Hospital of Wenzhou Medical University (No. L-2019-08), and informed consent given by all patients and/or their guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roupakias S, Sinopidis X, Tsikopoulos G, et al. Dimercaptosuccinic acid scan challenges in childhood urinary tract infection, vesicoureteral reflux and renal scarring investigation and management. Minerva Urol Nefrol 2017;69:144-52. [PubMed]
- Lim R. Vesicoureteral reflux and urinary tract infection: evolving practices and current controversies in pediatric imaging. AJR Am J Roentgenol 2009;192:1197-8. [Crossref] [PubMed]
- Morello William, La Scola Claudio, Alberici Irene, et al. Acute pyelonephritis in children. Pediatr Nephrol 2016;31:1253-65. [Crossref] [PubMed]
- Tekgül S, Riedmiller H, Hoebeke P, et al. EAU guidelines on vesicoureteral reflux in children. Eur Urol 2012;62:534-42. [Crossref] [PubMed]
- Lee BF, Chiou YY, Chuang CM, et al. Evolution of differential renal function after acute pyelonephritis. Nucl Med Commun 2002;23:1005-8. [Crossref] [PubMed]
- National institute for health and clinical excellence (nice) guideline. Urinary tract infection in children: diagnosis, treatment, and long term management. Available online: www.nice.org.uk/nicemedia/pdf/cg54fullguideline.pdf
- Basiratnia M, Noohi AH, Lotfi M, et al. Power Doppler sonographic evaluation of acute childhood pyelonephritis. Pediatr Nephrol 2006;21:1854-7. [Crossref] [PubMed]
- Stogianni A, Nikolopoulos P, Oikonomou I, et al. Childhood acute pyelonephritis: comparison of power Doppler sonography and Tc-DMSA scintigraphy. Pediatr Radiol 2007;37:685-90. [Crossref] [PubMed]
- Halevy R, Smolkin V, Bykov S, et al. Power Doppler ultrasonography in the diagnosis of acute childhood pyelonephritis. Pediatr Nephrol 2004;19:987-91. [Crossref] [PubMed]
- Bykov S, Chervinsky L, Smolkin V, et al. Power Doppler sonography versus Tc-99m DMSA scintigraphy for diagnosing acute pyelonephritis in children: are these two methods comparable. Clin Nucl Med 2003;28:198-203. [Crossref] [PubMed]
- Farhat W, Traubici J, Sherman C, et al. Reliability of contrast enhanced sonography with harmonic imaging for detecting early renal scarring in experimental pyelonephritis in a porcine model: preliminary results. J Urol 2002;168:1114-7. [Crossref] [PubMed]
- Majd M, Nussbaum Blask AR, Markle BM, et al. Acute Pyelonephritis: Comparison of Diagnosis with 99mTc-DMSA SPECT, Spiral CT, MR Imaging, and Power Doppler US in an Experimental Pig Model. Radiology 2001;218:101-8. [Crossref] [PubMed]
- Kawashima A, LeRoy AJ. Radiologic evaluation of patients with renal infections. Infect Dis Clin North Am 2003;17:433-56. [Crossref] [PubMed]
- Preda I, Jodal U, Sixt R, et al. Normal dimercaptosuccinic acid scintigraphy makes voiding cystourethrography unnecessary after urinary tract infection. J Pediatr 2007;151:581-4. [Crossref] [PubMed]
- Hitzel A, Liard A, Véra P. J Nucl Med 2002;43:27-32. [PubMed]
- Mandell GA, Eggli DF, Gilday DL, et al. Procedure guideline for renal cortical scintigraphy in children. Society of Nuclear Medicine. J Nucl Med 1997;38:1644-6. [PubMed]
- Linné T, Fituri O, Escobar-Billing R, et al. Functional parameters and 99mtechnetium-dimercaptosuccinic acid scan in acute pyelonephritis. Pediatr Nephrol 1994;8:694-9. [Crossref] [PubMed]
- Hitzel A, Liard A, Dacher JN, et al. Quantitative Analysis of 99mTc-DMSA During Acute Pyelonephritis for Prediction of LongTerm Renal Scarring. J Nucl Med 2004;45:285-9. [PubMed]
- Shaikh N, Borrell JL, Evron J, et al. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev 2015;1:CD009185. [PubMed]
- Williams GJ, Macaskill P, Chan SF, et al. Absolute and relative accuracy of rapid urine tests for urinary tract infection in children: a meta-analysis. Lancet Infect Dis 2010;10:240-50. [Crossref] [PubMed]
- Ammenti A, Cataldi L, Chimenz R, et al. Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow-up. Acta Paediatr 2012;101:451-7. [Crossref] [PubMed]
- Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics 2014;133:e1121-7. [Crossref] [PubMed]
- Kangari G, Esteghamati M, Ghasemi K, et al. Predictive accuracy of urinary β2-microglobulin for kidney injury in children with acute pyelonephritis. Iran J Kidney Dis 2015;9:19-24. [PubMed]
- Mohkam M, Karimi A, Habibian S, et al. Urinary N-acetyl-beta-D-glucosaminidase as a diagnostic marker of acute pyelonephritis in children. Iran J Kidney Dis 2008;2:24-8. [PubMed]
- Jéquier S, Jéquier JC, Hanquinet S. Acute childhood pyelonephritis: predictive value of positive sonographic findings in regard to later parenchymal scarring. Acad Radiol 1998;5:344-53. [Crossref] [PubMed]
- Chiou YY, Wang ST, Tang MJ, et al. Renal Fibrosis: Prediction from Acute Pyelonephritis Focus Volume Measured at 99mTc Dimercaptosuccinic Acid SPECT. Radiology 2001;221:366-70. [Crossref] [PubMed]
- Hansson S, Dhamey M, Sigström O, et al. Dimercapto-succinic acid scintigraphy instead of voiding cystourethrography for infants with urinary tract infection. J Urol 2004;172:1071-3; discussion 1073-4. [Crossref] [PubMed]


