
Dynamic change of Mycoplasma pneumoniae pneumonia in hospitalized children in a general hospital: a 3-year retrospective analysis
Introduction
Mycoplasma pneumoniae (MP) is a major cause of community-acquired pneumonia (CAP). In recent years, the incidence and severity of Mycoplasma pneumoniae pneumonia (MPP) cases have been increasing (1-3), posing new challenges to clinical diagnosis and treatment. Untreated or severe MPP can affect multi-organs injury, such as the brain, heart, peripheral nervous system, skin, and kidneys as well as hemolytic anemia. In general, children are more susceptible to MP infections than adults. This is aggravated by the fact that they’re often surrounded by large groups of other infectious children. As such, children may be at a higher risk for MPP than adults. Compared with children specialist hospital, most of children admitted to the pediatric wards in general hospitals have respiratory infections, especially CAP, and a large proportion of them are MPPs. CAP children, especially MPP children, are the main population of pediatric inpatients in general hospital. Generally, the basic diagnosis and treatment of MPP in children is clear. Some studies have analyzed clinical features and epidemiological characteristics of MPP in children (4-6). However, the epidemiology and economic burden of hospitalized CAP due to MP is still poorly understood and may change year by year. Therefore, in this study we analyzed the relevant features of the dynamic changes of 2,011 pediatric patients with CAP over 1 year of age admitted to our center within the past 3 years, with an attempt to inform the clinical diagnosis and management of MPP.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tp-20-149).
Methods
Subjects
A total 2011 of CAP children aged 1–16 years hospitalized at the Department of Pediatrics of Peking University Third Hospital from January 1, 2017 to December 31, 2019 were enrolled in this retrospective analysis. The diagnostic criteria for CAP included the following: (I) pneumonia occurring in otherwise healthy children before hospitalization or within 48 hours of hospitalization; (II) with respiratory sign/symptoms including fever, cough, increased respiratory rate, difficulty breathing, inspiratory retraction of the chest wall, wet rales, tubular breath sounds, and others; and (III) abnormal changes in chest imaging. MPP was further diagnosed among these CAP patients by using the passive agglutination (PA) method (Fujirebio, Japan) (1,2). An MP infection was confirmed if the MP antibody titer increased or decreased by 4 times or more during the recovery period or acute period.
The inclusion criteria for enrolled cases were: (I) the age range of children between 1 year to 16 years; (II) hospitalized children who meet the above CAP diagnostic criteria; (III) hospitalized children who had done test for MP infection. The exclusion criteria were: (I) hospitalized children who are younger than 1 year and older than 16 years; (II) hospitalized children who were diagnosed with nosocomial pneumonia.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of our center (Ethical approval number: IRB00006761-M2020097). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Diagnosis and treatment standards
The severity of CAP or MPP (mild or severe) was evaluated according to the Guidelines for the Management of Community Acquired Pneumonia in Children (2013 edition) (7). The assessment of intrapulmonary and extrapulmonary complications was based on the Criteria for Diagnosis and Treatment of Community-acquired Pneumonia in Children (2019 edition) (8). The use (or not) of flexible bronchoscopy was based on the Guidelines for the Management of Community-acquired Pneumonia in Children (2013 edition) (7), and the 2015 Expert Consensus on the Diagnosis and Treatment of Mycoplasma pneumoniae Pneumonia in Children (9).
Data collection and grouping
The clinical data including age, sex, seasonal distribution of MPP, duration of hospital stay, etiologies, severity of disease, complications, use of flexible bronchoscopy, and use of heated and humidified high-flow oxygen therapy were collected from electronic medical record information system by blind method. According to their ages, these patients were divided into an early childhood group (1–3 years), preschool group (4–6 years), and school-age or older group (7–16 years). Also, based on the pathogens, they were divided into MPP group and non-MPP group.
The differences of the above indicators in the CAP group and MPP group were compared across 3 consecutive years (2017, 2018, and 2019); also, these differences were compared between the MPP group and non-MPP group.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software. Measurement data with skewed distribution were described by median (M) and interquartile range (P25, P75), and count data were expressed as cases and percentages. Chi-square test, Kruskal-Wallis nonparametric test, and Mann-Whitney U test were used for inter-group comparisons. A P value of <0.05 was considered statistically significant.
Results
Clinical features of CAP children
A total of 2,011 pediatric inpatients were diagnosed with CAP in three years. These children included 1,014 males and 997 females, with a male to female ratio of 1.02:1.00; the median age was 4 years (range, 2–7 years). Among these children, 824 were in the early childhood group, 569 were in the preschool group, and 618 were in the school-age or older group. The proportion of CAP patients significantly increased in the 3 consecutive years (P<0.05). It was highest in 2017 for the early childhood group and highest in 2018 for the preschool group, and the differences were statistically significant for both groups (both P<0.05); CAP incidence showed a rising trend over these 3 years, but the difference was not statistically significant (P>0.05). The incidence of complications, incidence of severe CAP, use of flexible bronchoscopy, and use of high-flow oxygen treatment were not statistically different over these 3 years (all P>0.05) (Table 1). CAP was caused by a single pathogen in 1,998 patients (99.3%) and by 2 pathogens in 13 cases (0.7%). All CAP children were successfully cured and discharged, and no death was noted.
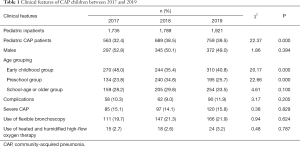
Full table
Overall clinical features of MPP children
Of 2,011 CAP patients, 825 MPP cases (41%) were confirmed over these 3 years, with a median age of 6 years (range, 4–8 years). The male-to-female ratio was not significantly different between the MPP group and non-MPP group (χ2=3.62, P>0.05). The positive rate of MPP was 22.3% (184/824), 43.4% (247/569), and 63.8% (394/618) in the early childhood group, preschool group, and school-age or older group (χ2=252.33, P<0.05). Further pairwise comparisons also showed statistically significant differences (all P<0.05), and the positive rate was highest in the school-age or older group and lowest in the early childhood group. Compared with the non-MPP group, the MPP group showed significant difference in seasonal incidences (P<0.05; Chi-square test). Further Chi-square tests between these two groups showed the incidences of MPP were significantly higher in Q3 and Q4 than in Q1 (both P<0.05); Q2 had the lowest incidence, and the difference was statistically significant (χ2=5.64, P<0.05); the difference between Q3 and Q4 was not statistically significant (χ2=1.20, P>0.05) (Table 2). Compared with non-MPP group, MPP group had a significantly increased incidence of severe pneumonia, incidence of complications, and use of flexible bronchoscopy (all P<0.05). The use of heated and humidified high-flow oxygen therapy was not significantly different between these two groups (P>0.05) (Table 3).

Full table
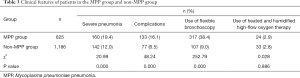
Full table
Dynamic changes of clinical features in the MPP group over 3 years
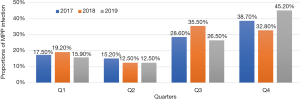
The positive rate of MPP and male-to-female ratio showed no significant change from 2017 to 2019 (both P>0.05), although the difference in the age composition was statistically significant (P<0.05). Further inter-group comparisons by using chi-square test showed that the number of MPP patients increased significantly in 2018 in the early childhood group (χ2=11.32, P<0.05) and in 2019 in the school-age or older group (χ2=9.313, P<0.05). The changes in the incidence of complications, severe CAP, use of flexible bronchoscopy, and use of heated and humidified high-flow oxygen therapy were not statistically significant across these 3 years in MPP patients (all P>0.05) (Table 4). The seasonal distribution of MPP also showed no significant change over 3 years (χ2=12.178, P>0.05) (Figure 1). The distribution of MP infection in different months over 3 years was further compared, which showed the incidence of MP infection significantly differed across the different months in 2018 (χ2=39.539, P<0.05) and reached its peak in August (MPP cases accounted for 60.3% of all CAP inpatients). In 2019, the incidence of MP infection also significantly differed across the different months (χ2=45.698, P<0.05) and reached its peak in November (MPP cases accounted for 64% of all CAP inpatients) (Figure 2).
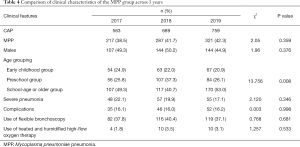
Full table
Dynamic changes in economic indicators in CAP and MPP children
Over these 3 years, the median hospital stay was 5 days (range, 3–7 days) in CAP children, which significantly decreased from 5 days (range, 3–6 days) in 2017 to 5 days (range, 4–7 days) in 2018 and 5 days (range, 3–7 days) in 2019 (z=6.295, P<0.05); for MPP children, the median hospital stay was 5 days (range, 4–8 days) in 2017, 6 days (range, 4–8 days) in 2018, and 6 days (range, 4–8 days) in 2019, showing no significant difference (P>0.05); for non-MPP children, the median hospital stay was 5 days (range, 3–6 days) in 2018, 4 days (range, 3–6 days) in 2017, and 4 days (range, 3–6 days) in 2019, showing significant difference (z=6.068, P>0.05). The median hospital stay was 6 days (range, 4–8 days) in the MPP group and 4 days (range, 3–6 days) in the non-MPP group (z=−11.131, P<0.05) (Figure 3).

The total hospitalization costs (5,869 yuan in 2017, 6,829 yuan in 2018, and 7,071 yuan in 2019), examination fees, non-medication treatment costs, and medication treatment costs increased year by year among CAP children in 3 consecutive years, and the differences were statistically significant (the z values were 35.24, 46.79, and 9.64, respectively; P<0.05). Compared with the non-MPP group, the MPP group had a significantly larger increase in total hospitalization costs, examination fees, non-medication treatment costs, and medication treatment costs (the z values were −14.82, −14.85, and −12.821, respectively; P<0.05). Dynamic comparisons inside the MPP group showed that the total hospitalization costs, examination fees, and non-medication treatment costs significantly increased each year (the z values were 11.51 and 15.20; both P<0.05), whereas the medication treatment costs showed no significant change [1,696.21 yuan (1,163.37, 2,785.15) in 2017, 2,003.18 yuan (1,351.18, 2,851.89) in 2018, and 2033.40 yuan (1,221.14, 3,051.06) in 2019] (z=4.81, P>0.05).
Discussion
The results from the current study show that CAP patients account for about one-third of the pediatric hospitalized children in general hospitals, and the proportion of CAP patients has risen over the past 3 years. Although young children (1–3 years) remain the most susceptible age group to CAP, the proportion of the school-age or older group is gradually increasing. This may be explained by the fact that the incidence of MPP is also increasing among school-age children; in addition, the school-age children and adolescents currently have a lower resistance to CAP, mainly due to various social factors such as high school stress and small living spaces. Despite the increase in CAP prevalence, the proportion of severe CAP, the incidence of complications, and the use of flexible bronchoscopy and oxygen therapy did not increase accordingly, and all the patients were cured and discharged without difficulty, suggesting that the diagnosis and treatment capabilities have also progressed and have lowered the incidences of severe CAP and complications.
Our current study showed that MPP accounted for 41% of all hospitalized CAP children. The incidence of MPP and the proportion of MPP cases among CAP patients have been rising over the past 3 years, but the difference was not statistically significant. This might be due to the single-center design and small sample size of our current study but might also suggest that the number of MPP patients have not increased dramatically over the past years. We also found that school-age children and adolescents had a highest incidence rate of MPP, which reached 63.8%. An Indian study showed that 59.5% of children ≥5 years were positive for Mycoplasma pneumoniae infection (10), which was similar to our current finding; a recent study in the United States also reported a similar result (11). Mycoplasma pneumoniae typically spreads through droplets, and school-age children and adolescents are more susceptible to Mycoplasma pneumoniae infection as they spend more time in closed or semi-closed learning environments. Therefore, the learning and living environments of school-age children and adolescents should be improved if conditions allow. MPP showed no gender difference in our current study; however, two previous studies in two areas in China reported the incidence of MPP to be higher in girls than in boys, which might be explained by the difference of the age of the studies and the sources of patients: both studies were based on the clinical data collected 5 years ago, and all the cases were from the respiratory departments of children’s hospitals (12,13).
Our current study also demonstrated that there was no seasonal variation in the incidence of CAP in the non-MPP group; in contrast, the incidence of MPP was highest in the Q3 and Q4 (with no statistically significant difference between these two quarters). Although MPP may occur year round, it is more common in the winter and spring. However, our current study revealed that the incidence of MPP in Q3 was higher than that in Q1 but did not differ from that in Q4. Further analysis showed that the higher incidence of MPP in Q3 might be linked to the increased MPP patients in the third quarter of 2018. It was reported that MPP outbreaks occurred in several elementary schools in the Haidian and Shunyi districts of Beijing in August 2018 (14,15), supporting the results of our current study. We also found that MPP was highly active in Q4 in 2017 and 2019, which was consistent with the high prevalence of MPP in winter reported by other studies (16). As a result, the proportion of MPP cases in Q3 of 2018 accounted for two-thirds of all MPP cases in 2018, suggesting an MPP outbreak might have occurred in Q3 of 2018, which also supports the epidemic cycles of MP infection. The previous epidemics of MP infection occurred in 1984, 1988, 2006, 2010, and 2015, respectively, and the epidemic reached its peak approximately every 3–7 years (17,18). Since the proportion of preschool children remarkably increased among MPP children in 2018, the preschool children were the main affected population during the MPP outbreak in Q3 of 2018.
In addition, the MPP group had a higher proportion of severe pneumonia cases, and the incidence of complications was higher than that in the non-MPP group; however, the need for heated and humidified high-flow oxygen therapy was not significantly different between these two groups, suggesting dyspnea was not a major problem in children with severe MPP. According to the 2013 and 2019 China Guidelines for the Management of Community-acquired Pneumonia in Children, pulmonary infiltrate on chest radiographs or lung computed tomography (CT) is one of the diagnostic criteria for severe CAP (7,8). After MP infects the human body, it causes diseases through direct damage and/or immune-mediated injuries. As the risk population of MPP, school-aged children and adolescents have a relatively mature immune system and thus will experience severe systemic inflammatory response to MP infection and are more likely to suffer from conditions such as segmental and lobar consolidations of the lungs (19). Accordingly, these patients are more likely to meet the diagnostic criteria for severe CAP. Compared with the 2013 guidelines, the 2019 revision describes more accurately the criteria for lung infiltrate of severe CAP, has stricter requirements for the scope of lung infiltrate and is easier to apply; thus, the new guidelines are particularly instructive for severe MPP.
In addition, although studies have shown that the proportion of severe MPP is rising, our current analysis of data over 3 years did not find an increasing trend of severe MPP, which might be explained by the proactive use of macrolides and flexible bronchoscopy in our center. In fact, up to 38.4% of MPP patients underwent flexible bronchoscopy in our current study, which, as shown in another study (20), effectively shortened the disease course of MPP and improved the prognosis. The proportion of MP with resistance to antibiotics has increased annually since the first macrolide-resistant MP strain was isolated in 1968. It was reported that the proportion of the macrolide-resistant MP strain reached up to 90% between 2008 and 2012 (21). The positive rates of drug-resistant MP strains are also rising in Japan (22), which increases the difficulty of MPP treatment. Nevertheless, macrolides remain the preferred empirical treatment for CAP (especially MPP). Shah et al. (23) suggested that there was a window for detection of MP after infection; before the detection results became positive, early use of macrolides was associated with a short duration of fever and lower risk of serious complications. According to the clinical practice guidelines released by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America, macrolides are the preferred antibiotics for CAP (24). Meanwhile, the incidence of intrapulmonary and extrapulmonary complications was similar across these 3 years in MPP children, which was consistent with our finding that severe MPP did not increase over 3 years. Therefore, active empirical use of macrolides based on the epidemiological features of MPP helps to lower the severity of MPP.
Our current study showed that the average hospital stay of CAP children has decreased annually; however, the average hospitalization days showed no significant change from 2017 to 2019 in the MPP group but significantly decreased in the non-MPP group, suggesting the decrease in the average hospital stay of non-MPP children was the main cause of the decreased hospital stay among CAP children. Also, there was an increasing trend in the cost of CAP treatment. The treatment costs were higher in the MPP group than in the non-MPP children, which seemed to be related to the higher proportions of severe cases, complications, and use of flexible bronchoscopy in the MPP group. However, although the cost of MPP treatment has increased annually, the proportions of severe cases, complications, and use of flexible bronchoscopy showed no rising trend in the MPP group, suggesting that the annual increase in total hospitalization costs and examination/non-medication treatment costs cannot be solely attributed to the increased proportion of the use of flexible bronchoscopy. Therefore, the analysis of the average hospitalization days and treatment costs suggests that the average hospitalization period has not increased significantly during the increase in the incidence of MPP, which is an important component of CAP; meanwhile, the proportion of examinations and non-pharmacological treatments has increased, indicating that the early use of macrolides and flexible bronchoscopy may be a more reasonable and economical health protocol for MPP management.
According to our results, it may be effective to implement the following strategies to prevent MPP and decrease the severe MPP: (I) by improving the living and learning environment of high-risk population (school-age children and adolescents) in high-risk seasons, especially in the third and the fourth quarters; (II) by actively administrating macrolides antibiotics in high-risk population and in high-risk seasons even if MP test has not been carried out or the result of MP test has not been returned.
The limitations of this study were: (I) data coming from single center; (II) data which was retrospectively collected; (III) CAP children aged from 28 day to 1 year which were not enrolled; (IV) variables which were not very detailed.
In summary, CAP is the most common disease among pediatric inpatients in general hospitals, with MP being the main pathogen. The epidemiological features of MPP are age- and season-specific and may change annually. The proportions of severe pneumonia, complications, and use of flexible bronchoscopy, along with the length of hospital stay and hospitalization costs, are greater in MPP children than in non-MPP children. Therefore, general hospitals should develop integrated clinical quality control programs for MPP children. Active empiric treatments may shorten hospital stay, reduce hospitalization costs, and thus optimize the allocation of medical resources.
Acknowledgments
Funding: This project was funded by a research grant from Beijing Natural Science Foundation (No. S170003 to YX, No. S160004 to XT) and Cross Research Seed Fund of Peking University Medicine (BMU2020MX010 to YX).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-149
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-20-149
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-149). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of our center (Ethical approval number: IRB00006761-M2020097). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Waites KB, Xiao L, Liu Y, et al. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin Microbiol Rev 2017;30:747-809. [Crossref] [PubMed]
- Rogozinski LE, Alverson BK, Biondi EA. Diagnosis and treatment of Mycoplasma pneumoniae in children. Minerva Pediatr 2017;69:156-60. [PubMed]
- Gao LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China:2006 to 2016. Epidemiol Infect 2019;147:e192. [Crossref] [PubMed]
- Kim JH, Kwon JH, Lee JY, et al. Clinical features of Mycoplasma pneumoniae coinfection and need for its testing in influenza pneumonia patients. J Thorac Dis 2018;10:6118-27. [Crossref] [PubMed]
- Søndergaard MJ, Friis MB, Hansen DS, et al. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS One 2018;13:e0195288. [Crossref] [PubMed]
- Wang K, Gill P, Perera R, et al. Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev 2012;10:CD009175. [PubMed]
- Respiratory Medicine Group of Pediatrics Branch of Chinese Medical Association & Editorial Board of Chinese Journal of Pediatrics. Guidelines for the Management of Community Acquired Pneumonia in Children (2013 revision). Chin J Pediatr 2013;51:745-52.
- Expert panel. Guidelines on diagnosis and treatment of community-acquired pneumonia in children (2019 update). Clin Edu General Pract 2019;17:771-7.
- Respiratory Medicine Group of Pediatrics Branch of Chinese Medical Association & Editorial Board of Chinese Journal of Pediatrics. Guidelines for the management of community-acquired pneumonia in children (2015 revision). J Appl Clin Pediatr 2015;30:1304-8.
- Kumar S, Garg IB, Sethi GR. Mycoplasma pneumoniae in Community-Acquired Lower Respiratory Tract Infections. Indian J Pediatr 2018;85:415-9. [Crossref] [PubMed]
- Kutty PK, Jain S, Taylor TH, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis 2019;68:5-12. [PubMed]
- Yan C, Sun HM, Zhao HQ, et al. Epidemiological characteristics of Mycoplasma pneumoniae infection in hospitalized children in Beijing: 10-year retrospective analysis. Chin J Appl Clin Pediatr 2019;34:1211-4.
- Zhang XX, Ji W, Gu WJ, et al. Epidemiological analysis of Myeoplasma pneumoniae infection in children with respiratory tract diseases in Suzhou area from 2005 to 2014. Clin J Infect Dia 2015;33:594-8.
- Chen CZ, Cai W, Wang JG, et al. Survey on Mycoplasma pneumoniae pneumonia outbreak in a primary school in Haidian District, Beijing. J Preventive Med Inform 2020;36:10-4.
- Zhang WZ, Zhang SJ, Wang QY, et al. Outbreak of macrolide-resistant mycoplasma pneumoniae in a primary school in Beijing, China in 2018. BMC Infect Dis 2019;19:871. [Crossref] [PubMed]
- Yamazaki T, Kenri T. Epidemiology of Mycoplasma pneumoniae Infections in Japan and Therapeutic Strategies for Macrolide-Resistant M. pneumoniae. Front Microbiol 2016;7:693. [Crossref] [PubMed]
- Lee E, Kim CH, Lee YJ, et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect Dis 2020;20:132. [Crossref] [PubMed]
- Qu J, Yang C, Bao F, et al. Epidemiological characterization of respiratory tract infections caused by mycoplasma pneumoniae during epidemic and post-epidemic periods in North China, from 2011 to 2016. BMC Infect Dis 2018;18:335. [Crossref] [PubMed]
- Lee YH, Seo H, Cha SI, et al. A case of pseudomembranous tracheitis caused by Mycoplasma pneumoniae in an immunocompetent patient. Ann Transl Med 2019;7:205. [Crossref] [PubMed]
- Shao MK, Zhou Y, Du K, et al. Treatment of children with severe Mycoplasma pneumonia by electronic bronchoscope. Chin J Nosocomiology 2016;26:4735-7.
- Wang Y, Ye Q, Yang D, et al. Study of two separate types of macrolide-resistant Mycoplasma pneumoniae outbreaks. Antimicrob Agents Chemother 2016;60:4310-4. [Crossref] [PubMed]
- Ando M, Morozumi M, Adachi Y, et al. Multilocus Sequence Typing of Mycoplasma pneumoniae, Japan, 2002-2016. Emerg Infect Dis 2018;24:1895-901. [Crossref] [PubMed]
- Shah SS. Mycoplasma pneumoniae[M]. Long SS, Prober CG, Fischer M, eds. Principles and practice of pediatric infectious diseases. 5th ed. Philadelphia: Elsevier Inc., 2018;1023-7.
- Bradley JS, Byington CL, Shah SS, et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age:clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:e25-76. [Crossref] [PubMed]
(English Language Editor: J. Gray)



