Pediatric hepatoblastoma: diagnosis and treatment
Introduction
Hepatoblastoma (HBL) is the most common primary liver tumor in children and is usually diagnosed during the first 3 years of life. Most HBLs are sporadic, but some are associated with constitutional genetic abnormalities and malformations, such as the Beckwith-Wiedemann syndrome and familial adenomatous polyposis (1,2). Over the last three decades, the annual incidence of HBL in children has gradually increased (3). Extremely premature babies with a birth weight of less than 1 kilo have been reported to have a greatly increased risk of developing HBL. The increased survival rates of these premature babies might account for the increased annual incidence of HBL.
Diagnosis of HBL
The most common sign is abdominal distension or abdominal mass. Some children present with abdominal discomfort, generalized fatigue, and loss of appetite, due to tumor distension or secondary anemia. Children with a ruptured tumor usually present with vomiting, symptoms of peritoneal irritation, and severe anemia. Rare cases manifest precocious puberty/virilization due to β-human chorionic gonadotropin (hCG) secretion by the tumor. Serum alpha-fetoprotein (AFP) is the most important clinical marker for HBL, and remains the key clinical marker of malignant change, response to the treatment, and relapse. However, there are some variants of both HBL and hepatocellular carcinoma (HCC) that have low or normal AFP levels (4,5). These variants, such as rhabdoid tumor, may have distinct histological features and worse prognosis.
Abdominal ultrasonography usually reveals a large mass in liver, sometimes with satellite lesions and areas of hemorrhage within the tumor. The most useful diagnostic modality is multiphase computed tomography (CT) or magnetic resonance imaging (MRI). Helical CT findings of hypervascular lesions in the liver with delayed contrast excretion are highly suggestive of a malignant liver tumor. Histological diagnosis of a tumor specimen is essential, although some investigators believe that biopsy may not be necessary for young children (6 months to 3 years) with a very high AFP level (6); in addition, avoiding a biopsy theoretically reduces the risks of tumor seeding or dissemination. The Japanese Study Group for Pediatric Liver Tumors (JPLT) strongly recommends that liver tumors of children should be treated after definitive diagnosis of a biopsy specimen, except in urgent life-threatening circumstances such as tumor invasion of the right atrium or tumor rupture (7).
Segmental assessment of the extent of the tumor and its relationship to the main hepatic vessels is of utmost importance when planning the intensity of chemotherapy and eventual surgery. In Europe, the Childhood Liver Tumor Study Group of the International Society of Pediatric Oncology (SIOPEL) has developed the preoperative evaluation of the tumor extent (PRETEXT) staging system, which appears to be a valuable tool for risk stratification (8); although the system has not been formally evaluated for prognostic accuracy. Formal staging of the tumor should include chest and brain CT. The risk stratification system proposed by the Children’s Hepatic Tumors International Collaboration (CHIC), which will be described later, is shown in Table 1.
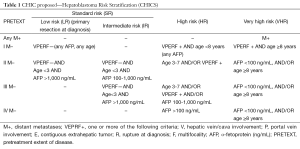
Full table
In childhood hepatic tumors, clinically relevant histologic subtypes are also being incorporated into risk stratification systems as well as into the PRETEXT staging system and distant metastasis. The common HBL subtypes are as follows: epithelial, mesenchymal, fetal, and embryonal. Some HBL variants include cholangioblastic or teratoid components or a macrotrabecular growth pattern (9). Fibrolamellar HCC is a distinct clinical and histological variant of pediatric HCC. The histopathological subtypes are the major prognostic factors of pediatric liver tumors, including HCC. The Children’s Oncology Group (COG) has found that patients with completely resected tumors (stage I) with pure fetal histology have an excellent outcome (10), while both the SIOPEL and the COG investigators found that HBL patients presenting with low AFP levels (<100 ng/mL) and/or with small cell undifferentiated (SCUD) histology had a poor outcome, regardless of the PRETEXT staging system (4,5). The SCUD histology has not been reported by Japanese investigators. Rhabdoid tumors, which show loss of SMARCB1/INI1 expression on immunohistochemistry, should be included in the differential diagnosis of patients with tumors and low AFP levels (11). In 2011, an International Pathology Symposium was held to perform a collaborative histopathological review of pediatric liver tumors to work towards a consensus classification, with the eventual aim of developing a common treatment-stratification system. This symposium proposed the current standardized, histopathological meaningful classification of pediatric liver tumors (12).
Treatments for HBL
Before 1980, children with malignant hepatic tumors could only be cured by complete surgical resection of tumors. At present, complete tumor resection remains the cornerstone of definitive cure for HBL and offers the only realistic chance of long-term disease-free survival (13-15). The introduction of effective chemotherapeutic regimens in the 1980s resulted in an increased number of patients who could ultimately undergo tumor resection and also reduced the postoperative recurrence rate. Moreover, modern surgical techniques based on the segmental anatomy of the liver and whole hepatectomy plus liver transplantation have also led to increased numbers of resectable patients and have markedly improved the prognosis of these patients. Therefore, the combination of surgery and chemotherapy is an essential therapeutic strategy for HBL. The COG and JPLT studies have approved primary resection for children with resectable tumors, especially PRETEXT I or II cases. However, SIOPEL studies have not permitted the used of primary resection.
Recently, international collaboration study should be required for prompt clinical trials. CHIC was formed to focus on international global cooperation for investigations of pediatric malignant hepatic tumors, including HBL. The leading multicenter groups in CHIC are JPLT, SIOPEL, GPOH (German Paediatric Oncology and Haematology Society) and COG. Risk stratification in these trials was based on individual special classification of stage, metastasis, and histology in each trial (16). These CHIC members have incorporated their unique data into a common database, which now includes the retrospective data of all children treated in eight separate multicenter HBL trials performed between 1985 and 2008 (1,605 patients) (13-15,17-23).
The identification and development of new prognostic stratifications has led to novel treatments for high-risk patients and treatment reduction for low-risk patients, who do not need therapy intensification but need to avoid the delayed effects and unnecessary toxicities associated with treatment (24). Since childhood cancers are leading the way in the use of risk-adapted therapeutic strategies (25,26), collaborative research based on common risk adaptations and chemoprotective therapy for toxicity will have many benefits throughout the field of pediatric oncology. Although the analysis of CHIC database is still being debated, the therapeutic strategies used in global studies will be proposed using the risk stratification system proposed by CHIC (Table 1).
Standard-/low-risk patients
Patients with a single localized tumor involving at most three segments of the liver (PRETEXT I, II, III) can safely undergo complete surgical resection because of recently developed surgical instruments and anatomical evaluation using imaging modalities. Standard-risk patients are those patients with PRETEXT I, II, and III tumors and no extrahepatic features [hepatic vein/cava involvement (V), portal vein involvement (P), contiguous extrahepatic tumor (E), rupture at diagnosis (R), and multifocality (F)] or distant metastasis (M) (Table 1).
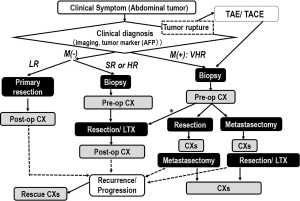
The treatment algorithm is shown in Figure 1. JPLT and COG have permitted primary hepatectomy for patients with PRETEXT I and II tumors, but SIOPEL has recommended preoperative chemotherapy for every patient, which is followed by tumor resection (19,27-29) or liver transplantation (27), and a short course of postoperative chemotherapy for most cases. The current consensus based on CIHC analysis is that an initial resection can be performed for PRETEXT I or II tumors if the tumor is located at least 1 cm from the middle hepatic vein and the bifurcation of the portal vein. Preoperative chemotherapy should be performed for other situations. Cisplatin-and-anthracycline-based chemotherapy was used as the first-line regimen in European (SIOPEL) and Japanese (JPLT) studies. This regimen improved the survival rates of patients with resectable tumors (15,17). The SIOPEL-1 study (PLADO) used four triweekly preoperative and two postoperative cycles of cisplatin (CDDP) and doxorubicin (DOXO), and resulted in an overall response rate of 82% and 5-year event-free survival (EFS) of 66% (28). The JPLT studies, using the same four preoperative and two postoperative cycles of CDDP and pirarubicin (THP-ADM), and the COG studies, using the same cycles of CDDP, fluorouracil (5-FU), and vincristine, resulted in almost similar survival rates. CDDP monotherapy recently achieved similar rates of complete resection and survival among children with resectable tumors (15). Therefore, CDDP monotherapy will be first-line chemotherapy for these standard HBLs. A trial is underway that evaluates combination therapy using CDDP and sodium thiosulphate to reduce the late effects of CDDP, especially ototoxicity.
High-risk (HR) patients
These patients are those with unresectable tumor at diagnosis and/or associated with so-called “combi factors” without distant metastasis. The combi factor is a combination of the cross sectional imaging components including macrovascular involvement retrohepatic vena cava or all three hepatic veins (V); macrovascular involvement portal bifurcation or both right and left portal veins (P); contiguous extrahepatic tumor (E); multifocal disease (F); and spontaneous rupture (R) at diagnosis. V, P, E, F and R where a patient is categorized as positive when at least 1 of the components is present in HR. In addition, CHIC analysis found that older patients (≥3 years old at diagnosis) and patients with ruptured or multifocal tumors at diagnosis had unfavorable outcomes. Therefore, these patients were included as high-risk patients, even if their tumor was resectable (30,31). Conventional preoperative chemotherapy used in the PLADO, CITA, and C5V trials for patients with PRETEXT IV unresectable tumors resulted in tumors that could be resected by hepatectomy in some patients; but the outcome of patients with unresectable tumors at diagnosis remained unsatisfactory. Therefore, in SIOEPL studies, chemotherapy for HR-HBL was gradually intensified by the addition of carboplatin (SuperPLADO study) or high-dose CDDP (SIOPEL-4 study) with shortened intervals between chemotherapy cycles (31,32). In COG studies, the HR-HBL regimen was also intensified by addition of DOXO and high-dose CDDP (C5VD) (33). The SIOPEL-4 and COG approaches, based on CDDP intensification, improved the survival of children with HR-HBL, including those with lung metastases. Therefore, at present, CDDP intensification protocols are being evaluated for patients with HR-HBL, although the toxicity and late complications of these treatment protocols remain unclear.
In addition, orthotropic liver transplantation has improved the outcome for some patients with unresectable tumors (PRETEXT IV tumors or tumors with portal or hepatic vein involvement) (34). Although the timing of liver transplantation and the role of rescue transplantation therapy remain controversial, consultation for liver transplantation should be performed for high-risk patients during the early stages of preoperative chemotherapy.
Very high-risk patients (metastatic HBL)
There is strong agreement that patients who present with lung metastases have a poor prognosis. In addition, CHIC analysis revealed that older patients (≥8 years old at diagnosis) and patients with low AFP levels (<100 ng/mL) have unfavorable outcomes. Therefore, these patients were included as very high-risk patients even if their tumor had not metastasized (35,36).
Conventional chemotherapy was usually ineffective for these very high-risk patients, with survival rates under 40% in the previous SIOPEL-1 and JPLT-1 studies (8,18). Moreover, resection of lung metastases has been effective for some patients with metastatic tumors. The surgical guideline for lung metasectomy should be necessary to improve outcome of the patient with lung metastasis in future. CDDP intensification therapy such as that used in the SIOPEL-4 protocol seems to be effective for these patients. The 3-year survival of metastatic HBL cases who underwent the SIOPEL-4 protocol was approximately 80% (31). To decrease recurrence, a consolidation regimen should be considered for these very high-risk patients. In addition, new molecular targeting therapy using vincristine and irinotecan will be investigated by COG.
For some of these metastatic patients whose metastatic lesions respond to these approaches, liver transplantation may be an indication. Therefore, carefully planned combination therapy using dose-intensified chemotherapy and surgical approaches that include metastasectomy and liver transplantation should be required to treat the very high-risk HBL patient.
Other therapies
Transarterial embolization (TAE) is used to control peritoneal hemorrhage in patients with ruptured tumors. Since primary resection for such rupture cases results in poor outcome, interventional control for hemorrhage is required for more successful treatment of these HBL patients.
It is well known that the normal liver receives blood from two sources, the hepatic artery and portal vein. Malignant liver tumors, including HBL, are mainly fed by the hepatic artery. Therefore, transarterial chemoembolization (TACE) is tumor selective. JPLT has used TACE instead of systemic chemotherapy as a clinical trial (17). CDDP and anthracycline have infused with particles that are used for embolization. The effect of TACE for HBL seemed to be equivalent to that of the patients who were treated by systemic chemotherapy and might be less toxic in comparison with systemic chemotherapy. Therefore, TACE is one of the effective procedures for pediatric liver tumors. However, administering TACE to children is somewhat difficult and requires general anesthesia. Verification of the efficacy of TACE for patients with standard-risk HBL requires additional clinical trials.
Future plans
To obtain consensus for universal risk stratification and treatment of pediatric HBL, CHIC has created the largest database to date of patients with this rare cancer. The CHIC based classification system described in this review is being incorporated into a new risk-based cooperative international trial, the Pediatric Hepatoblastoma International Therapeutic Trial (PHITT), a joint venture of global collaboration that includes SIOPEL, COG, and JPLT.
Acknowledgements
Funding: This review was partially supported by Grants-in-Aid for Scientific Research (A) (No. 13313631 and 13370806) from the Ministry of Education, Culture, Sports, Science, and Technology and for Cancer Research by that from the Ministry of Health, Labor, and Welfare of the Government of Japan (No. H26-Kakushintekigun-Ippan-067, and H26-Iryougijyutu-Ippan-008).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cohen MM Jr. Beckwith-Wiedemann syndrome: historical, clinicopathological, and etiopathogenetic perspectives. Pediatr Dev Pathol 2005;8:287-304. [PubMed]
- Garber JE, Li FP, Kingston JE, et al. Hepatoblastoma and familial adenomatous polyposis. J Natl Cancer Inst 1988;80:1626-8. [PubMed]
- Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer 2008;112:416-32. [PubMed]
- De Ioris M, Brugieres L, Zimmermann A, et al. Hepatoblastoma with a low serum alpha-fetoprotein level at diagnosis: the SIOPEL group experience. Eur J Cancer 2008;44:545-50. [PubMed]
- Trobaugh-Lotrario AD, Tomlinson GE, Finegold MJ, et al. Small cell undifferentiated variant of hepatoblastoma: adverse clinical and molecular features similar to rhabdoid tumors. Pediatr Blood Cancer 2009;52:328-34. [PubMed]
- Czauderna P, Otte JB, Aronson DC, et al. Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer 2005;41:1031-6. [PubMed]
- Nitta A, Hisamatsu S, Fukuda H, et al. Cardiopulmonary arrest on arrival in an infant due to ruptured hepatoblastoma. J Pediatr 2012;160:351. [PubMed]
- Perilongo G, Shafford E, Plaschkes J, et al. SIOPEL trials using preoperative chemotherapy in hepatoblastoma. Lancet Oncol 2000;1:94-100. [PubMed]
- López-Terrada D, Zimmermann A. Current issues and controversies in the classification of pediatric hepatocellular tumors. Pediatr Blood Cancer 2012;59:780-4. [PubMed]
- Malogolowkin MH, Katzenstein HM, Meyers RL, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children’s Oncology Group. J Clin Oncol 2011;29:3301-6. [PubMed]
- Al Nassan A, Sughayer M, Matalka I, et al. INI1 (BAF 47) immunohistochemistry is an essential diagnostic tool for children with hepatic tumors and low alpha fetoprotein. J Pediatr Hematol Oncol 2010;32:e79-81. [PubMed]
- López-Terrada D, Alaggio R, de Dávila MT, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol 2014;27:472-91. [PubMed]
- Ortega JA, Douglass EC, Feusner JH, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol 2000;18:2665-75. [PubMed]
- Pritchard J, Brown J, Shafford E, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach--results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol 2000;18:3819-28. [PubMed]
- Perilongo G, Maibach R, Shafford E, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 2009;361:1662-70. [PubMed]
- Le Bail B. Pathology: a pictorial review. A selected atlas of paediatric liver pathology. Clin Res Hepatol Gastroenterol 2012;36:248-52. [PubMed]
- Hishiki T, Matsunaga T, Sasaki F, et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2: report from the JPLT. Pediatr Surg Int 2011;27:1-8. [PubMed]
- Sasaki F, Matsunaga T, Iwafuchi M, et al. Outcome of hepatoblastoma treated with the JPLT-1 (Japanese Study Group for Pediatric Liver Tumor) Protocol-1: A report from the Japanese Study Group for Pediatric Liver Tumor. J Pediatr Surg 2002;37:851-6. [PubMed]
- Perilongo G, Shafford E, Maibach R, et al. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology--SIOPEL 2. Eur J Cancer 2004;40:411-21. [PubMed]
- Fuchs J, Rydzynski J, Von Schweinitz D, et al. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumor Study HB 94. Cancer 2002;95:172-82. [PubMed]
- Häberle B, Bode U, von Schweinitz D. Differentiated treatment protocols for high- and standard-risk hepatoblastoma--an interim report of the German Liver Tumor Study HB99. Klin Padiatr 2003;215:159-65. [PubMed]
- Katzenstein HM, Rigsby C, Shaw PH, et al. Novel therapeutic approaches in the treatment of children with hepatoblastoma. J Pediatr Hematol Oncol 2002;24:751-5. [PubMed]
- Katzenstein HM, Chang KW, Krailo M, et al. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: a report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children’s Oncology Group. Cancer 2009;115:5828-35. [PubMed]
- McDermott U, Settleman J. Personalized cancer therapy with selective kinase inhibitors: an emerging paradigm in medical oncology. J Clin Oncol 2009;27:5650-9. [PubMed]
- Walterhouse D, Watson A. Optimal management strategies for rhabdomyosarcoma in children. Paediatr Drugs 2007;9:391-400. [PubMed]
- Gustafson WC, Matthay KK. Progress towards personalized therapeutics: biologic- and risk-directed therapy for neuroblastoma. Expert Rev Neurother 2011;11:1411-23. [PubMed]
- Czauderna P. Hepatoblastoma throughout SIOPEL trials - clinical lessons learnt. Front Biosci (Elite Ed) 2012;4:470-9. [PubMed]
- Perilongo G, Brown J, Shafford E, et al. Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer 2000;89:1845-53. [PubMed]
- Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer 2012;59:818-21. [PubMed]
- von Schweinitz D. Hepatoblastoma: recent developments in research and treatment. Semin Pediatr Surg 2012;21:21-30. [PubMed]
- Zsiros J, Brugieres L, Brock P, et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol 2013;14:834-42. [PubMed]
- Zsíros J, Maibach R, Shafford E, et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol 2010;28:2584-90. [PubMed]
- Meyers RL, Tiao G, de Ville de Goyet J, et al. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 2014;26:29-36. [PubMed]
- Meyers RL, Tiao GM, Dunn SP, et al. Liver transplantation in the management of unresectable hepatoblastoma in children. Front Biosci (Elite Ed) 2012;4:1293-302. [PubMed]
- Czauderna P, Lopez-Terrada D, Hiyama E, et al. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 2014;26:19-28. [PubMed]
- Watanabe K. Current chemotherapeutic approaches for hepatoblastoma. Int J Clin Oncol 2013;18:955-61. [PubMed]


