Vitamin D intake in young children with acute lower respiratory infection
Introduction
Acute lower respiratory infection (ALRI), such as pneumonia and bronchiolitis, is the leading cause of mortality worldwide in children less than age 5 years and represents the most common reason for hospital admission of children within this age group (1,2). Risk factors for the development of ALRI include low birth weight, non-exclusive breastfeeding, incomplete immunization, indoor air pollution, crowding, parental smoking, and chronic disease (3). It is also hypothesized that the reduction of ultraviolet-B (UVB) radiation exposure during winter is associated with decreased vitamin D production that could account partly for the increased prevalence of ALRI during winter months (4).
Vitamin D insufficiency has been implicated in a number of immune related illnesses including multiple sclerosis, type 1 diabetes, and cancer (5,6), as well as respiratory diseases such as asthma (7-9). In developing countries there is an association between the risk of ALRI and both rickets and subclinical vitamin D deficiency in children (10-14). The same association has not been demonstrated elsewhere, but vitamin D deficiency has been linked to ALRI severity in Canada and the Middle East (15,16). This variability could be secondary to differences in study population and design. Additionally, serum 25 hydroxy vitamin D is likely influenced by acute illness and medical intervention, perhaps making it a less reliable measure of pre-illness vitamin D status (17,18).
Vitamin D (cholecalciferol) is either produced in the skin through UVB radiation or absorbed via the intestine following dietary intake. Given the uncertain reliability of 25(OH)D levels during illness, the objective of this analysis was to determine if vitamin D intake is associated with ALRI during the winter months, when UVB exposure is low. The vitamin D intake of children hospitalized with pneumonia or bronchiolitis was compared to an unmatched control group in the same age range without ALRI. This data was collected as part of a larger study examining vitamin D intake, serum 25(OH) levels and ALRI (15).
Materials and methods
Subjects and case definition
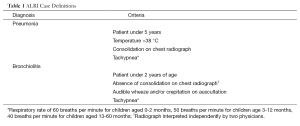
Participants were recruited at the Royal University Hospital, Saskatoon, Saskatchewan, Canada from November 2007 to May 2008. Potential study participants included patients less than age 5 years admitted with a diagnosis of either bronchiolitis or pneumonia. These were defined by clinical and radiographic criteria (Table 1). The control population comprised a convenience sample of children less than age 5 years without respiratory symptoms who were receiving care at the Royal University Hospital’s ambulatory, emergency or in-patient units. There were no exclusion criteria. The University of Saskatchewan’s Biomedical Research Ethics Board approved the study protocol.
Full table
Demographic and questionnaire information
A research nurse or recruiting physician recorded date of enrollment, age, sex, and medication utilization obtained from health records. At enrollment, caregivers were asked to complete a questionnaire documenting the presence of known ALRI risk factors including: cigarette smoke exposure, ethnicity, breastfeeding history, immunization status, daycare attendance, history of prematurity, birth weight and co-morbid disease. Ethnicity was self-reported as Caucasian, Aboriginal/Métis, Black, Asian or Other. Adequate breastfeeding was defined as having been breastfed for at least 6 months. An incomplete immunization status was defined as none or partial vaccination in children over 2 months of age. Prematurity was defined as birth at a gestational age less than 36 weeks. As an indicator of socioeconomic status, the household person-to-bedroom ratio was calculated as previously described (19). Caregivers also provided their postal code, from which the first three characters were used to categorize the region of residence as northern Saskatchewan (latitude 53 to 59 degrees) or southern Saskatchewan (latitude 49 to 52 degrees).
Daily vitamin D intake was estimated using a food frequency questionnaire; parents reported their child’s dietary intake of milk, fish, egg and supplements in the week before enrolment. Daily vitamin D intake from milk was calculated using both the quantity and type of milk. Concentrations of vitamin D were assigned at 40 IU/L for breast milk, 500 IU/L for formula, 400 IU/L for soy and cow milk, 50 IU per egg, and 250 IU for a serving of fish (20-23). For those breastfed children without a known quantity of milk intake, the participant’s weight was used to approximate a daily maintenance fluid allotment. For those children who were taking a supplement containing vitamin D, the amount of vitamin D in the supplement was utilized; when the brand of supplement was not known a value of 400 IU was assigned. As it has been proposed that vitamin D requirements might change with age, the vitamin D intake of participants was calculated as IU/kg (24).
Sample size calculation and statistical analysis
The sample size was estimated using a comparison of means, and 10 IU/kg was chosen as the minimum detectable clinically significant difference in vitamin D intake between ALRI and control groups. A standard deviation of 20 IU/kg was conservatively estimated based on the National Health and Nutrition Examination Survey (25). When setting the alpha error at 0.05 and the power at 0.9, a sample size in excess of 85 subjects per group was calculated.
Data were recorded in a computerized database [Statistical Package for the Social Sciences (SPSS) Version 18, Chicago, IL]. All data collected over the six month period were anonymized for the purposes of this study. SPSS was used for data analyses. Results are presented as frequencies; percentages for categorical variables or means with standard deviations (SD) for continuous variables for cases and controls separately. Differences in proportions were assessed by means of the 2-tailed Fisher exact test/Chi-square analysis. A bivariate logistic regression analysis was conducted to determine the association between vitamin D intake and ALRI. Based on the model building procedure, univariate variables with P<0.20 were selected for the multivariate model. For comparisons, odds ratio (OR) and 95% confidence interval (CI) were calculated.
Results
At the end of the 6-month study period, the parents and caregivers of 197 children consented to participate (105 ALRI, 92 Control). The participation rate was 95%; one ALRI subject who met inclusion criteria refused to participate and of the 93 control patients who were approached, one declined to consent. Seventeen of those who consented did not fully complete their questionnaire (8 ALRI, 9 Controls) but any available data was used in the analysis. Among the 105 ALRI cases, 55 had bronchiolitis and 50 had pneumonia. Among these, 16 (15.5%) had disease severe enough to require admission to the Pediatric Intensive Care Unit. The diagnoses or presenting complaints for the control group participants are shown in Table 2. Of the 92 control subjects, 48 were recruited from the inpatient ward, 31 from the emergency department and 13 from outpatient clinics.

Full table
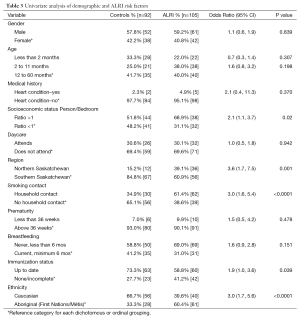
The ALRI group and control groups were not significantly different with respect to sex (approximately 60% males and 40% females in both groups) and age at enrollment (13.4±13.8 and 13.8±15.2 months respectively). All but six participants identified their ethnicity as Caucasian or Aboriginal/Métis (Asian 3, Black 2, Other 1). The ethnicity categories were therefore collapsed into Caucasian and Non-Caucasian, and separate analyses considered the Caucasian and Aboriginal/Métis groups. ALRI subjects were significantly more likely to be Non-Caucasian (OR 3.5, 95% CI: 1.9,6.4) compared to Caucasian. They were also more likely to have self-reported Aboriginal/Metis ancestry compared to Caucasian (OR 3.0, 95% CI: 1.7, 5.6).
Finally, ALRI subjects were significantly more likely to live in Northern Saskatchewan (OR 3.6, 95% CI: 1.7, 7.5), to have a person-to-bedroom ratio greater than 1 (OR 2.1, 95% CI: 1.1, 3.7), to have a household smoking contact (OR 3.0, 95% CI: 1.6, 5.4), and an incomplete immunization status (OR 1.9, 95% CI: 1.0, 3.6). The presence of a heart condition, daycare attendance and breastfeeding were not significantly associated with ALRI (Table 3).
Full table
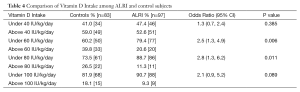
The mean vitamin D intake of control subjects was 60 IU/kg/d compared to 48 IU/kg/d in the ALRI group (P=0.09). The standard deviation for vitamin D intake was 44 IU/kg/d. In order to determine if a certain level of vitamin D intake is associated with the risk of ALRI, vitamin D intake was dichotomized as greater and equal, or less than 40, 60, 80 or 100 IU/kg/d. Bivariate analysis was performed at each level (Table 4). A daily intake of less than 60 IU/kg/d was significantly associated with ALRI (OR 2.5, 95% CI: 1.3, 4.9) as was an intake of less than 80 IU/kg/d (OR 2.8, 95% CI: 1.3, 6.2).
Full table
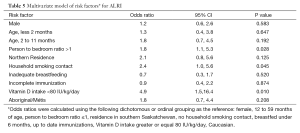
In the multivariate model, a person-to-bedroom ratio greater than one (OR 1.8, 95% CI: 1.1, 5.3), a household smoking contact (OR 2.4, 95% CI: 1.0, 5.6) and vitamin D intake less than 80 IU/kg/d (OR 4.9, 95% CI: 1.5, 16.4) remained significantly associated with ALRI (Table 5). Substituting the Aboriginal/Métis variable with the collapsed ethnicity variable of Non-Caucasian did not significantly change the results of the multivariate analysis. When a vitamin D intake of less than 60 IU/kg/d was substituted into the model, only person-to-bedroom ratio (OR 2.3, 95% CI: 1.0,4.9) and vitamin D intake were statistically significant (OR 2.7, 95% CI: 1.0, 7.3).
Full table
Finally, post-hoc analysis was performed examining the pneumonia and bronchiolitis groups separately. A vitamin D intake of less than 80 IU/kg/d was found to be significantly associated with pneumonia (OR 7.9, 95% CI: 1.8, 35.5), but not bronchiolitis (OR 1.7, 95% CI: 0.7, 4.0).
Discussion
The objective of this study was to determine if an association existed between vitamin D intake and ALRI in young children during the winter months when UVB radiation is low. In a group of children aged less than 5 years receiving hospital based care, those with ALRI had a lower vitamin D intake. This result approached statistical significance, but the analysis was underpowered due to a larger than expected standard deviation in the study population. However, bivariate analysis using different thresholds for vitamin D intake showed that intakes of less than 60 and 80 IU/kg/day were both significantly associated with ALRI.
Aboriginal ancestry, residence in northern Saskatchewan, lower socioeconomic status, exposure to household smoking, and incomplete immunizations were also associated with an increased risk of ALRI. As ethnicity and socio-economic status have previously been associated with vitamin D status, these factors were controlled for in the multivariate model (26-29). When controlling for these factors, a vitamin D intake of less than 80 IU/kg/d (2 mcg/kg/d) remained significant and was associated with a 4-fold increase in the risk of ALRI compared to children with an intake of greater than 80 IU/kg/d. Similarly, although not as statistically robust, the threshold level of 60 IU/kg/d remained associated with ALRI after controlling for other risk factors. These data suggest a threshold vitamin D intake, around 60 to 80 IU/kg/d, above which there might be some protection against ALRI.
In addition to its role in influencing bone health, vitamin D is also now known to have important effects on immune and inflammatory responses. Vitamin D modulates white blood cell proliferation, maturation and cytokine expression through cognate receptors on lymphocytes and macrophages (30-32). Vitamin D receptor signaling contributes to the expression of antimicrobial peptides and toll-like receptors, molecules important for innate defense against both viruses and bacteria (33,34). These mechanisms might at least partly explain why rickets, as well as subclinical vitamin D deficiency, is associated with ALRI in children from developing countries (10-14). Furthermore, in a randomized controlled trial, children aged 1-36 months with pneumonia who received a single oral dose of 100,000 IU of D3 were less likely to have a repeat episode in the 90 days after supplementation (35).
In North America, the link between ALRI and vitamin D has not been as clear. The authors’ own study examining ALRI and 25(OH) vitamin D levels did not show a statistically significant association, but a trend was observed with the pneumonia patients having lower 25(OH)D levels compared to those with bronchiolitis (15). In addition, patients with severe disease were more likely to have lower serum concentrations of 25(OH)D. Correlation between vitamin D intake and serum 25(OH)D levels was poor in our sample, possibly because patients had 25(OH)D collected at variable times during their illness, depending on when they were scheduled to have blood work. Serum 25(OH)D levels may have been impacted by medications, IV fluids and the illness itself (17,18). Vitamin D intake may be a reasonable alternative in estimating pre-illness vitamin D status. Interestingly, two recent publications measuring 25 hydroxyvitamin D in cord blood showed that infants with lower levels were more likely to develop respiratory tract infection during the first year of life (36,37).
Another Canadian study examining young children with bronchiolitis did not show lower vitamin D levels in this group, although they were found to have a specific vitamin D receptor (VDR) polymorphism resulting in an under active VDR (19,38). It has been suggested that this polymorphism may need higher levels of 25(OH)D, and thus higher vitamin D intake, to overcome the hypoactive VDR and provide protection against bronchiolitis. These findings might explain why in our subgroup analysis, a vitamin D intake of less than 80 IU/kg/d was associated with pneumonia but not bronchiolitis. The role of vitamin D in ALRI might not be limited to serum 25(OH)D levels, and may instead be related to the level of intake, the study population (race and genetic make-up) and the disease process (bronchiolitis vs. pneumonia).
The Canadian Pediatric Society recommends that infants receive 400 IU/d of vitamin D in the first year of life (800 IU north of the 55th parallel), and the American Academy of Pediatrics recommends this amount for children of all ages (24,39). This level of supplementation should be sufficient to prevent vitamin D deficient rickets; however it may not be adequate to protect against other illnesses or achieve the recommended minimum serum 25(OH)D levels of 75-80 nmol/L (24,40). For example, in a recent Canadian study, more than 50% of infants aged less than 2 years had a serum 25(OH)D level less than 80 nmol/L despite having a vitamin D intake of 400 IU/d (41).
The results of the present study suggest that apart from young infants (<6 kg), 400 IU/d would be inadequate to achieve a level of intake above 80 IU/kg/day, and that a more appropriate vitamin D dose might be better estimated according to weight. In fact, in their position statement, the Canadian Pediatric Society has suggested that infants and young children may need a vitamin D intake up to 2.5 mcg/kg/d (100 IU/kg/d) in order to optimize their serum 25(OH)D levels (24). Furthermore, a study correlating vitamin D intake and serum levels suggested that vitamin D supplementation in infants should consider their rapid body weight increment (42). Finally, the 2011 Institute of Medicine recommendations for vitamin D intake consider benefits for bone health only but do suggest different dosing based on age. In addition to recommended daily intakes of 400 and 600 IU for children under and over 1 year of age, the IOM provides an upper limit for vitamin D intake based on 6 different pediatric age ranges (43).
There are a number of limitations to the present study, the first of which relates to the inherent imprecision of dietary recall. In addition, a number of assumptions were made in calculating participants’ vitamin D intake, including the milk intake of breastfed infants, and the vitamin D content of breastmilk, and of supplements if they were not named. Sun exposure was also not considered, although vitamin D synthesis through skin exposure during fall and winter months is negligible above latitudes greater than 40 degrees (41).
Further research is needed to better correlate vitamin D intake with serum 25(OH)D levels in children of different ages. Additional studies should further explore the relationship of vitamin D intake and 25(OH)D levels with various disease states. Specifically, the results of this study should be confirmed. Finally, consideration should be given to the idea that optimal daily dosing of vitamin D may be better described according to a child’s weight.
Acknowledgements
The authors would like to thank the following people for their contributions: the children and families who participated in the study, Brenda Andreychuk and Marie Penner for their assistance with patient recruitment and Loren Matheson for preparing study materials. The authors would also like to acknowledge financial support from: (I) Department of Pediatrics, University of Saskatchewan, Canada (II) Royal University Hospital Foundation (III) Pediatric Rheumatic Disease Research Laboratory, University of Saskatchewan (IV) Canadian Arthritis Network.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Canadian Institute of Child Health. The Health of Canada’s Children, 3rd Edition. Ottawa, ON: Canadian Institute of Child Health, 2000.
- Mulholland K. Global burden of acute respiratory infections in children: implications for interventions. Pediatr Pulmonol 2003;36:469-74. [PubMed]
- Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008;86:408-16. [PubMed]
- Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect 2006;134:1129-40. [PubMed]
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353-73. [PubMed]
- Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA 2002;287:3116-26. [PubMed]
- Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85:788-95. [PubMed]
- Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr 2007;85:853-9. [PubMed]
- Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010;126:52-8.e5.
- Muhe L, Lulseged S, Mason KE, et al. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 1997;349:1801-4. [PubMed]
- Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr 2004;50:364-8. [PubMed]
- Banajeh SM, al-Sunbali NN, al-Sanahani SH. Clinical characteristics and outcome of children aged under 5 years hospitalized with severe pneumonia in Yemen. Ann Trop Paediatr 1997;17:321-6. [PubMed]
- Wayse V, Yousafzai A, Mogale K, et al. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 2004;58:563-7. [PubMed]
- Roth DE, Shah R, Black RE, et al. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr 2010;99:389-93. [PubMed]
- McNally JD, Leis K, Matheson LA, et al. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol 2009;44:981-8. [PubMed]
- Banajeh SM. Nutritional rickets and vitamin D deficiency--association with the outcomes of childhood very severe pneumonia: a prospective cohort study. Pediatr Pulmonol 2009;44:1207-15. [PubMed]
- Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr 2011;93:1006-11. [PubMed]
- Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care 2010;14:R216. [PubMed]
- Roth DE, Jones AB, Prosser C, et al. Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr 2009;63:297-9. [PubMed]
- Ala-Houhala M, Koskinen T, Parviainen MT, et al. 25-Hydroxyvitamin D and vitamin D in human milk: effects of supplementation and season. Am J Clin Nutr 1988;48:1057-60. [PubMed]
- Kamao M, Tsugawa N, Suhara Y, et al. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2007;859:192-200. [PubMed]
- Reeve LE, Chesney RW, DeLuca HF. Vitamin D of human milk: identification of biologically active forms. Am J Clin Nutr 1982;36:122-6. [PubMed]
- Holden JM, Lemar LE, Exler J. Vitamin D in foods: development of the US Department of Agriculture database. Am J Clin Nutr 2008;87:1092S-6S. [PubMed]
- Canadian Pediatric Society, First Nations and Inuit Health Committee [Principal author: Godel J]. Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatr Child Health 2007;12:583-98. [PubMed]
- Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 2005;135:2478-85. [PubMed]
- Marrie TJ, Carriere KC, Jin Y, et al. Hospitalization for community-acquired pneumonia in Alberta First Nations Aboriginals compared with non-First Nations Albertans. Can Respir J 2004;11:336-42. [PubMed]
- Stein EM, Laing EM, Hall DB, et al. Serum 25-hydroxyvitamin D concentrations in girls aged 4-8 y living in the southeastern United States. Am J Clin Nutr 2006;83:75-81. [PubMed]
- Weiler HA, Leslie WD, Krahn J, et al. Canadian Aboriginal women have a higher prevalence of vitamin D deficiency than non-Aboriginal women despite similar dietary vitamin D intakes. J Nutr 2007;137:461-5. [PubMed]
- Räsänen M, Kronberg-Kippilä C, Ahonen S, et al. Intake of vitamin D by Finnish children aged 3 months to 3 years in relation to sociodemographic factors. Eur J Clin Nutr 2006;60:1317-22. [PubMed]
- Bhalla AK, Amento EP, Clemens TL, et al. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab 1983;57:1308-10. [PubMed]
- Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol 1986;98:311-22. [PubMed]
- Abu-Amer Y, Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cell Immunol 1993;151:356-68. [PubMed]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770-3. [PubMed]
- Liu PT, Stenger S, Tang DH, et al. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 2007;179:2060-3. [PubMed]
- Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 2010;15:1148-55. [PubMed]
- Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011;127:e1513-20.
- Camargo CA Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011;127:e180-7. [PubMed]
- Roth DE, Jones AB, Prosser C, et al. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J Infect Dis 2008;197:676-80. [PubMed]
- Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, et al. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142-52. [PubMed]
- Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317-22. [PubMed]
- Roth DE. Bones and beyond: an update on the role of vitamin D in child and adolescent health in Canada. Appl Physiol Nutr Metab 2007;32:770-7. [PubMed]
- Pludowski P, Socha P, Karczmarewicz E, et al. Vitamin D supplementation and status in infants: a prospective cohort observational study. J Pediatr Gastroenterol Nutr 2011;53:93-9. [PubMed]
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53-8. [PubMed]


