
The effect of high-frequency oscillatory ventilation or airway pressure release ventilation on children with acute respiratory distress syndrome as a rescue therapy
Introduction
Children with acute respiratory distress syndrome (ARDS) who invasive mechanical ventilation experience high mortality. They are often rescued with modes of nonconventional mechanical ventilation. It includes high-frequency oscillatory ventilation (HFOV) and airway pressure release ventilation (APRV). ARDS is a common critical disease in pediatric intensive care units (PICUs) with a prevalence of 7.6% (1,2). Commonly, the effect of conventional comprehensive therapies, such as synchronized intermittent mandatory ventilation (SIMV), fluid management, and administration of medication, have been unsatisfactory with low oxygenation and high PaCO2 levels, which has resulted in high mortality in critically ill children (3). In theory, as part of a lung protection strategy, HFOV can be used to reduce induced injury (4,5) by delivering small tidal volumes at high rates (3–15 breaths/second) (6,7). HFOV is performed as elective and rescue therapy for ARDS in the PICU. It can optimize alveolar recruitment and lung volume, it can also improve oxygenation through the application of high flow rates and frequencies (900 cycles/minute) with low tidal volumes. A high and persistent medium airway pressure is maintained due to the minimal differences in expiratory and inspiratory pressures.
Some studies have shown that HFOV is effective in treating premature infants or neonates with respiratory failure (8,9). However, in recent years, some researchers, such as Gupta et al. (10) and Goffi and Ferguson (11), have presented the opposite viewpoint, finding that HFOV does not reduce mortality compared to ventilation in adults and may in fact be associated with worse outcomes in children.
In recent years, APRV has been considered as an alternate mode for refractory hypoxemia (12). Compared with SIMV, using lower peak pressures and inspiratory flow rates can prolong continuous positive airway pressure to recruit available lung units of varying time constants, and it also uses periodic time-cycled releases to facilitate CO2 clearance. The experience of using APRV is limited, but some studies suggest potential benefits, such as improved vasopressor requirement, cardiac output, and cardiorespiratory advantages of permissive spontaneous respiration throughout the ventilator cycle. In our department, HFOV and APRV are used as rescue therapy in children with acute respiratory failure after failure of SIMV in lung protection strategies. However, to our best knowledge, no study to support its use has been found (13,14). In this retrospective study, we aim to describe the effects of the application of HFOV and APRV as rescue ventilatory support in children with moderate and severe ARDS. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tp-19-178).
Methods
Design
A retrospective study of children with moderate and severe ARDS in the PICU was conducted from January 2014 to February 2019. Children who had been switched to APRV or HFOV after failure of SIMV were included. The trial was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Children’s Hospital of Zhejiang University School of Medicine (No. 2020-IRB-048) and informed consent was taken from all the patients.
Patient and data selection
Forty-seven ARDS patients between 1 month and 6 years old were recruited. They were divided into two groups: HFOV (SIMV switched to HFOV) group and APRV (SIMV switched to APRV) group. All children were transitioned from SIMV to either HFOV or APRV for 48 h or longer after failure of SIMV. There was no statistic difference between the two groups in terms of age, sex, body weight, or PRISM (Pediatric Risk of Mortality) III scores. The diagnosis of ARDS was established according to the conference in intensive care medicine (15). The application of HFOV or APRV was performed randomly in cases of failure of SIMV, no improvement in oxygenation, and retention of CO2. In addition to demographic data, the following items were recorded: arterial blood gases, ventilator settings, oxygenation index (OI), and PaO2/FiO2 (PF) ratio during the first 48 h of HFOV and APRV.
SMIV strategy
All patients were managed initially with SIMV using a Dräger Evita 4 ventilator (Drägerwerk AG & Co. KGaA, Lübeck, Germany) in the early stages of ARDS. Initiating conventional ventilation with a minimum of 5 cmH2O of positive end-expiratory pressure (PEEP) and 6–8 mL/kg of tidal volume and to attempt to reduce FiO2 to ≤0.6 is our institutional practice for ARDS. The inability to reduce FiO2 prompts escalation of PEEP and subsequent repeat efforts to reduce FiO2, with the goal to maintain peak inspiratory pressures (PIP) <30 cmH2O, oxygen saturation >88–90%, and permissive hypercapnia up to a PaCO2 of 55–60 mmHg, with maintenance of a pH >7.25–7.30. When patients suffered persistently elevated PIP (≥35 cmH2O), oxygenation difficulties (inability to decrease FiO2 to ≤0.60 despite increasing PEEP), or ongoing hypercarbia (PaCO2 ≥80 or pH <7.25) we prompted the consideration of changing the ventilation mode of HFOV or APRV as a rescue therapy.
APRV strategy
APRV would be initiated by selecting a peak pressure (Phigh) and inspiratory time (Thigh) to at least match the mean airway pressure (mPaw) being delivered by conventional ventilation, with stepwise increases in mPaw by adjusting Phigh or Thigh if we were unable to reduce FiO2 to ≤0.60. Our practice is setting low pressure (Plow) to 0 to facilitate rapid emptying and adjusting expiratory time (Tlow) to terminate at 1/2–3/4 of peak expiratory flow.
HFOV strategy
Patients were converted to HFOV in cases of failure of CMV through setting the mPaw to at least match that delivered by conventional ventilation, and with mPaw escalation until the FiO2 could be reduced to under 0.60. The amplitude was set to achieve oscillations visible in the pelvis. We set the initial frequency based on patient weight and age. Specifically, we used the following HFOV settings: a FiO2 of 0.4–1.0, an oscillatory frequency of 6–12 Hz, an inspiration time of 33% of the respiratory cycle, and a pressure amplitude (∆P) of 25–50 mbar. The oxygenation goal was oxygen saturation of ≥88% on FiO2 ≤0.60, and the ventilation goal was PaCO2 ≤60 with a pH ≥7.3. Muscle relaxants were given in combination with a sedative to patients who had acute deterioration of gas exchange during excessive spontaneous activity.
Data collection and definitions
We conducted a detailed retrospective review of all medical records. The review included demographics, admission diagnoses, indications for mechanical ventilation, length of mechanical ventilation, ventilator variables (including duration of APRV and HFOV, blood gas analysis, OI, and ventilator settings), vasopressor use in the first 48 h after transition (from SIMV to HFOV or APRV), and mortality.
The calculated measures of oxygenation were the PF ratio and the OI [(mPaw×FiO2×100)/PaO2]. The PF ratio and OI before transition are designated “PFpre” and “OIpre”, respectively; the values at 2 and 48 h after transition to HFOV are designated PF2h or PF48h and OI2h or OI48h.
Statistical analysis
Continuous variables were described as the means ± standard deviations when they were normally distributed, and median when they were skewed Categorical variables are reported as frequencies and percentages. Continuous data was compared using Student’s t-test or the Wilcoxon rank-sum test (16). Categorical data was compared using a two-tailed Fisher’s exact test or a Chi square test. P<0.05 was considered as statistically significant. Analyses were performed with SPSS 17.0 (IBM Corporation, New York, NY, USA).
Results
Patient characteristics
Forty-seven patients (26 males, 55%) with moderate and severe ARDS were transitioned from conventional ventilation (SIMV strategy) to either APRV or HFOV during the study period as shown in Table 1. The most common diagnoses were viral pneumonia and bacterial pneumonia; 16 and 14 patients with viral or bacterial pneumonia were transitioned to APRV and HFOV, respectively. All patients met the radiographic and oxygenation criteria for ARDS at the time of transition. No significant differences were observed with regard to age, length of SIMV, oxygenation, mPaw, PIP, and PaCO2 between the two groups before transition to APRV or HFOV. Three patients were eventually converted to extracorporeal membrane oxygenation (ECMO), and one patient survived (previously in the HFOV group). Patients with ARDS failed conventional ventilation and transitioned to APRV or HFOV at a median length of 1.9 days (1.94±0.73 days) of SIMV and at a median PIP of 35 cmH2O (35.26±2.56 cmH2O) before transitioning to APRV or HFOV (Table 2).

Full table
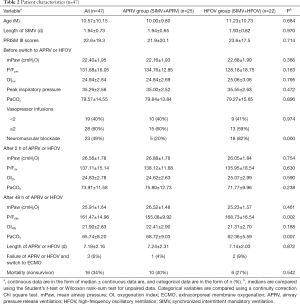
Full table
The mPaw at 2 h (26.56±1.78 cmH2O) and at 48 h after transition (25.91±1.64 cmH2O) was higher in both modes than the pre-transition value (22.40±1.95, P<0.01). The PF2h and PF48h in both modes were significantly improved compared to pre-transition values (P<0.001). Significant differences were observed in PF48h and PaCO2 at 48 h between the APRV and HFOV groups (P<0.01). The OI2h was not improved in either group (P>0.05), and the OI48h of the APRV and HFOV groups were substantially lower than the OIpre (P<0.001) in both groups. The OI of survivors in both cohorts improved over time.
The overall mortality was 34%, with no significant difference between the APRV and HFOV groups. Three patients (one APRV and two HFOV) transitioned to ECMO at a median of 3.5 [3–8] days after transition to APRV or HFOV and at a median of 6 [4–10] days after starting any mechanical ventilation. And the median OI at the time of ECMO cannulation was 36 [33–67] days. All three ECMO patients were cannulated while on HFOV. Two of these three patients died; the one survivor was cannulated to venovenous ECMO after 3 days of HFOV with a pre-ECMO OI of 38.
Ten of 25 patients (40%) with ARDS who transitioned to APRV died. Six of 22 patients (27%) who transitioned to HFOV died. We found no demographic or physiologic variable was associated with mortality before transition to either APRV or HFOV in the two groups. At 48 h after transition to both APRV and HFOV, the survivors had higher PF ratio, lower mPaw, and lower OI values (Table 3) than the non-survivors.
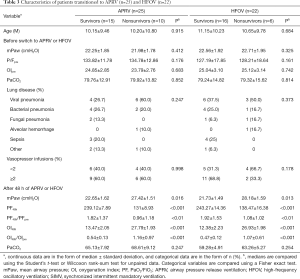
Full table
Discussion
In this study, pediatric patients with ARDS who transitioned to either APRV and HFOV had a high mortality rate (40% and 27%, respectively). Although the survival rates of the two groups show no significant difference, but the primary analysis indicates that the HFOV group may have more favorable oxygenation than the APRV group at an early stage. Survival was statistically associated with the improvement in oxygenation variables at 48 h after transition to APRV or HFOV. Pediatric patients failing SIMV transitioned to APRV or HFOV relatively early in the course of respiratory failure, with substantial oxygenation defects. The median mPaw before switching to APRV and HFOV was 22.40±1.95 cmH2O, which was somewhat lower than the pressures used to determine failure of conventional ventilation in a previous pediatric study (17) in which pediatric patients with respiratory failure were transitioned to HFOV at a median mPaw of 26 cmH2O. However, the present value is similar to the mPaw of 22 cmH2O at which 60 pediatric patients with an immunocompromised condition and ARDS were transitioned to HFOV or APRV (18). The relatively early transition may be associated with greater comfort with HFOV or APRV in our institution and an unwillingness to increase the peak inflating pressures of 35 cmH2O. At 2 and 48 h, the mPaw increased substantially with the improvement in the PF ratio but not in the OI. It suggested the increased alveolar recruitment at the cost of higher mPaw. No significant difference in mortality was observed between the two modes (40% in the APRV cohort and 27% in the HFOV cohort, P=0.542), and mortality was slightly lower than the 56% mortality rate of ARDS patients which was reported in a previous study (19). Patients in our study had well oxygenation, such as a low OI and high P/F, which may explain the lower mortality in our study compared to other previous reported data. Since this paper was a retrospective study, and the application of HFOV and APRV was a rescue therapy, we did not analyze the merely SIMV applied patients.
For patients who transitioned to either APRV or HFOV, the most useful predictors of mortality were measures of oxygenation at 48 h after transition as a fraction of the pre-transition values, which was reported as the PF48h/PFpre and OI48h/OIpre. In both the APRV and HFOV cohorts, the median OI48h of survivors was nearly 45% lower than the median OIpre, whereas in non-survivors, the OI48h and OIpre were nearly identical (Table 3). The discriminating values of PF48h/PFpre and OI48h/OIpre might reflect more highly recruitable lung tissue in the survivors. These patients experienced a much larger increase in the PF ratio at 48 h (>75% increase in the PF ratio on APRV, >90% increase on HFOV) with the increased mPaw than non-survivors. This result is corroborated by the relative improvements in OI (>45% reduction in OI on APRV, >50% reduction on HFOV). It suggests that survivors with more recruitability can improve their PF ratio at a relatively lower mPaw, in contrast to non survivors. PF48h/PFpre and OI48h/OIpre are also useful early markers to distinguish success or failure of APRV or HFOV. Therefore, this allows more invasive therapies to be instituted earlier in children with ARDS. To early detect the children with ARDS transitioned to either APRV or HFOV who have high risk of mortality will allow more directed use of potentially beneficial treatments, including prone positioning, extracorporeal support, or exogenous surfactant. The earlier transition potential to ECMO may bring benefits as prolonged time on any type of mechanical ventilation pre-ECMO has been related to higher mortality (20-22).
The relationship between the mortality and the improvement in the OI and PF ratio within 48 h after transition to APRV or HFOV should be further determined in prospective studies. Recent adult studies showed that the increased mortality (23) or no effect (24) of HFOV compared to conventional ventilation, calling into question the use of early oscillatory ventilation. In our study, children with ARDS were transitioned to APRV or HFOV with higher OIs and lower PF ratios than in either adult HFOV trial, and the two modes were used to “rescue” hypoxemia refractory to conventional ventilation. But given these adult studies results, the current use of HFOV as rescue ventilation seems justified.
This research had some limitations, first, it had a small sample size, and it was a retrospective observational study. Secondly, we designed a short follow-up time. Though the association between survival and improved oxygenation was robust, these results should be determined in a prospective study with larger population.
Conclusions
There was no significant difference in mortality rate between the two groups failing SIMV and transitioning to either APRV or HFOV. Improving the oxygenation at 48 h expressed as PF48h/PFpre and OI48h/OIpre can reliably discriminate survivors from non-survivors. Due to the small sample size, single center and retrospective study, considerably more research is needed before this conclusion can be verified.
Acknowledgments
Funding: The project was supported by Shanghai shenkang new transformation project (No. 16CR3085B) and the Shanghail Natural Science Foundation of China (No. 19ZR1432900).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tp-19-178
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-19-178
Peer Review File: Available at http://dx.doi.org/10.21037/tp-19-178
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-19-178). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Children’s Hospital of Zhejiang University School of Medicine (No. 2020-IRB-048) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farias JA, Frutos F, Esteban A, et al. What is the daily practice of mechanical ventilation in pediatric intensive care units? A multicenter study. Intensive Care Med 2004;30:918-25. [Crossref] [PubMed]
- Silva DC, Shibata AR, Farias JA, et al. How is mechanical ventilation employed in a pediatric intensive care unit in Brazil? Clinics (Sao Paulo) 2009;64:1161-6. [PubMed]
- Pinzon AD, Rocha TS, Ricachinevsky C, et al. High-frequency oscillatory ventilation in children with acute respiratory distress syndrome: experience of a pediatric intensive care unit. Rev Assoc Med Bras 1992;2013:368-74. [PubMed]
- Gerstmann DR, Minton SD, Stoddard RA, et al. The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics 1996;98:1044-57. [PubMed]
- Arnold JH, Hanson JH, Toro-Figuero LO, et al. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med 1994;22:1530-9. [Crossref] [PubMed]
- Ferguson ND, Slutsky AS. Point: High-frequency ventilation is the optimal physiological approach to ventilate ARDS patients. J Appl Physiol 1985;2008:1230-1. [PubMed]
- Muellenbach RM, Kredel M, Said HM, et al. High-frequency oscillatory ventilation reduces lung inflammation: a large-animal 24-h model of respiratory distress. Intensive Care Med 2007;33:1423-33. [Crossref] [PubMed]
- Zivanovic S, Peacock J, Alcazar-Paris M, et al. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med 2014;370:1121-30. [Crossref] [PubMed]
- Kessel I, Waisman D, Barnet-Grinnes O, et al. Benefits of high frequency oscillatory ventilation for premature infants. Isr Med Assoc J 2010;12:144-9. [PubMed]
- Gupta P, Green JW, Tang X, et al. Comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. JAMA Pediatr 2014;168:243-9. [Crossref] [PubMed]
- Goffi A, Ferguson ND. High-frequency oscillatory ventilation for early acute respiratory distress syndrome in adults. Curr Opin Crit Care 2014;20:77-85. [Crossref] [PubMed]
- Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med 2005;33:S228-40. [Crossref] [PubMed]
- Slee-Wijffels FY, van der Vaart KR, Twisk JW, et al. High-frequency oscillatory ventilation in children: a single-center experience of 53 cases. Crit Care 2005;9:R274-9. [Crossref] [PubMed]
- Faqih NA, Qabba'h SH, Rihani RS, et al. The use of high frequency oscillatory ventilation in a pediatric oncology intensive care unit. Pediatr Blood Cancer 2012;58:384-9. [Crossref] [PubMed]
- Saguil A, Fargo M. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician 2012;85:352-8. [PubMed]
- Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 2016;4:91. [Crossref] [PubMed]
- Ben Jaballah N, Khaldi A, Mnif K, et al. High-frequency oscillatory ventilation in pediatric patients with acute respiratory failure. Pediatr Crit Care Med 2006;7:362-7. [Crossref] [PubMed]
- Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med 2014;15:e147-56. [Crossref] [PubMed]
- Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 2005;293:470-6. [Crossref] [PubMed]
- Zabrocki LA, Brogan TV, Statler KD, et al. Extracorporeal membrane oxygenation for pediatric respiratory failure: survival and predictors of mortality. Crit Care Med 2011;39:364-70. [Crossref] [PubMed]
- Musick MA. Critical appraisal of Zabrocki et al: Extracorporeal membrane oxygenation for pediatric respiratory failure: survival and predictors of mortality. Crit Care Med 2011; 39:364-370. Pediatr Crit Care Med 2013;14:85-8. [Crossref] [PubMed]
- Minneci PC, Kilbaugh TJ, Chandler HK, et al. Factors associated with mortality in pediatric patients requiring extracorporeal life support for severe pneumonia. Pediatr Crit Care Med 2013;14:e26-33. [Crossref] [PubMed]
- Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795-805. [Crossref] [PubMed]
- Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013;368:806-13. [Crossref] [PubMed]


