
Retroperitoneal malignant triton tumor in an infant: a case report and literature review
Introduction
As an uncommon form of malignant peripheral nerve sheath tumor (MPNST), malignant triton tumors (MTTs) are differentiated by the presence of malignant rhabdomyosarcoma. They are most frequently encountered in patients with neurofibromatosis 1 (NF-1). This malignant subtype of schwannoma usually presents in younger people and affects males and females equally (1). The common sites of MTT are the head, neck, extremities, and trunk (1), although they are occasionally found in the buttock, viscera, retroperitoneum, and mediastinum. These cases are uncommon in pediatrics. To our knowledge, there have been only 33 reported cases to date (2-12). Here we present the case of an 8-month-old baby girl with a huge abdominal mass and review the histopathologic, cytogenic, treatment response, and prognostic findings of this rare case of MTT.
Case presentation
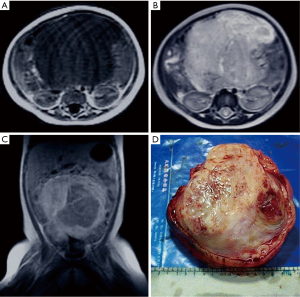
The patient was an 8-month-old baby girl who was admitted to hospital after an abdominal mass was found two days before. A physical examination revealed abdominal distension. A solid mass measuring approximately 10 cm × 11 cm × 8 cm was palpated, and no tenderness was observed. Laboratory tests showed white blood cell count (WBC) was 18.8×109/L, hemoglobin (HGB) was 78 g/L, and alpha-fetoprotein (AFP) was slightly positive. Ultrasound examination revealed a low echo-level space-occupying mass in the abdominal cavity. T1-weighted magnetic resonance imaging (MRI) indicated a huge hypo-intense tumor in the laparo-pelvic cavity. MRI showed the mass to be irregularly enhanced after an injection of gadolinium-diethylenetriamine pent acetic acid (Gd-DTPA). T2-weighted imaging (T2WI) revealed the tumor to be hyper-intense and inhomogeneous (Figure 1A,B,C). There was no significant perinatal or family history. On the basis of these findings, the proposed treatment was surgical resection. During the operation, a huge tumor with a remote hemorrhage in the abdominal cavity could be seen. It was a pedunculated tumor and came from the left iliac fossa in the retroperitoneal space, displacing the urinary bladder inferiorly. Since the left ovary and uterus were closely related to the tumor, they were also resected together with the tumor.
Pathologic study
On gross examination, the tumor was ovoid and gray, measuring approximately 14 cm × 12 cm × 9 cm. Its surface was smooth, and there was no obvious capsule. The cut surface showed a gray, solid, tough, weave-like mass, which was semitransparent in focal areas (Figure 1D). Microscopically, the tumor was composed of spindled cells and rhabdomyoblasts. The polygon rhabdomyoblasts had abundant cytoplasm, hyperchromatic nuclei with prominent nucleoli, and pathological mitotic figures.
Immunohistochemical staining was carried out to determine the origin of the cells and showed a positive reaction to neurofibroma (NF). The spindled cells and rhabdomyoblasts reacted with S-100 protein and desmin, respectively, and negatively reacted to actin (Figure 2A,B,C). The diagnosis of MTT was confirmed.
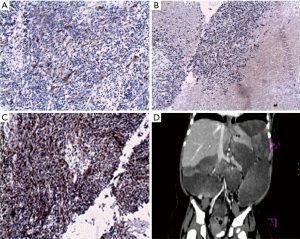
Two months after discharge, the patient was readmitted because of abdominal distention and diarrhea which lasted two weeks. Enhanced CT showed multiple low-density solid masses in the laparo-pelvic cavity, with uneven enhancement (Figure 2D). Recurrence and metastasis after surgery of retroperitoneal MTT were found to have occurred. Further therapy was refused by the patient’s parents. The patient died 20 days later, 5 hours after being readmitted to her local hospital for shock. An autopsy was not permitted.
Discussion
First reported by Masson (13) in 1932, MTTs acquired their name based on the work carried out by Locatelli on the “Triton” salamander (genus Triturus) (14). Locally transplanted the sciatic nerve onto the dorsal surface of a salamander, inducing neural and muscular components to grow and ultimately produce a supernumerary limb. Through this, a close relationship was shown to exist between these two tissues. The term ‘triton tumor’ was first used by Woodruff (15) in 1973 and histopathologic diagnostic criteria were established.
Woodruff suggested that malignant Schwann cells transform into striated muscle cells (15), resulting in the rhabdomyoblastic differentiation seen in MTTs, and direct evidence has since been shown to support this (16). However, Holimon and other scholars (17,18), bringing the embryogenesis and histogenesis of ectomesenchyme together, put forward the idea that both cellular components derived from neural crest cells. The neural crest is a unique group of embryonic cells which exists exclusively in vertebrates and is able to evolve a wide variety of cell types all over the body during embryonic life. These various types, including the mesenchymal and cartilage which make up the facial skeleton to neuronal cell constitute the peripheral sensory and autonomic nervous systems, also melanocyte and smooth muscle in the heart’s major arteries. Some presumably retain stem cell characteristics into maturity (19). Tumors comprising mixed neural crest derivatives have also been reported, further highlighting the capacity of neural crest cells to differentiate in multiple ways (20,21).
Although the etiology of MTT is unknown, 69% of cases are linked to Von Recklinghausen’s Neurofibromatosis and it is predominantly male and younger people who are affected. The incidence of MTT is in sharp contrast with sporadic cases of the disease, as these occur more frequently in older females (1).
Cytogenetic analyses of MTT have unearthed some karyotypic changes related to this tumor, but a consistent conclusion is yet to be reached. Overall, Chromosomes 17 and 22 were considered to be the possible substrates (22-27). As previously mentioned, MTT often occurs after NF-1, and the NF-1 gene, which has been mapped to 17q11.2 (28), is regarded as a tumor suppressor gene. In addition, the NF-2 gene, which has been mapped to 22q12, is also thought to have a tumor-suppressive function (24). Thus, these genes may serve important roles in the progression of a neurofibroma to a malignant schwannoma (22,25,29). Strauss et al. (30) detected strong nuclear immunoreactivity for p53 in MTT, both with or without NF-1, which may be associated with aggressive tumors (26,29). In a study involving 17 patients with 21 MPNSTs and MTTs [including 9 who had peripheral neurofibromatosis (NF1)], Bridge et al. (31) concluded that loss of chromosomal material was much more common than gain. Structural aberrations usually involve chromosomal bands or regions such as 1p31-36, 4q28-35, 7p22, 11q22-23, 19q13, 20q13, and 22q11-13. Haddadin et al. (26) suggested genes located at 7p22, 11p15, 7q36, 12p13, 13p11.2, 17q11.2 and 19q13.1 may serve in a pivotal role. The development of MTT can also feature other chromosomes, such as 8q (32).
CT or Magnetic Resonance Imaging (MRI) may prove useful for ascertaining a tumor’s location, size, and margin, and for assessing metastasis and response to therapy. However, MTT has no characteristic manifestation on CT or MRI. Spindle cells and rhabdomyoblasts are the typical markers in histopathological examination. Immunohistochemical staining is necessary as this helps to prove the origin of the cells.
Radical tumor excision, which is one of the recommended treatments for MTT, consists of radical tumor excision and removes as much of the wider normal tissue margin as possible. High-dose radiotherapy might prevent local recurrence and extend survival (1). The efficacy of neoadjuvant therapy and adjuvant chemotherapy has not yet been determined (26-29,31).
MTT has a poor prognosis, with a high rate of local recurrence [almost 100% in incompletely excised cases (1)] and a low survival rate less than 26% after five years (1,33,34)]. The location, grade and integrity of surgical procedures may have an influence on prognosis. MTT has a more favorable outcome when it is located in the head, neck or extremities compared with other sites including the retroperitoneum (28). Recurrence occurred for the patient in our study only two months after discharge, which is slightly longer than another reported retroperitoneal MTT (35). One study noted that NF-1-associated MTT has a poorer prognosis than sporadic forms (15-18,20). However, MTT patients’ poor survival rate cannot be entirely explained by tumor location and the association with neurofibromatosis. The high rate of histologic Grade 3 cases in MTT can better explain it aggression, as this sees a higher capacity for proliferation and increases the risk early metastases (1).
In conclusion, MTT is an aggressive and rare form of tumor with a grim prognosis, especially when it occurs the retroperitoneum of an infant. Radical tumor excision combined with radiotherapy or adjuvant or neoadjuvant chemotherapy is the recommended treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp.2020.03.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). All of the imaging examinations were agreed by the parents. Written informed consent was obtained from the patient’s parents for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brooks JS, Freeman M, Enterline HT. Malignant "triton" tumors. Natural history and immunohistochemistry of nine new cases with literature review. Cancer 1985;55:2543-9. [Crossref] [PubMed]
- Dimonitsas E, Liakea A, Sakellariou S, et al. An update on molecular alterations in melanocytic tumors with emphasis on Spitzoid lesions. Ann Transl Med 2018;6:249. [Crossref] [PubMed]
- Okur FV, Oguz A, Karadeniz C, et al. Malignant triton tumor of the pelvis in a 2-year-old boy. J Pediatr Hematol Oncol 2006;28:173-6. [Crossref] [PubMed]
- Brtko J, Sejnova D, Ondkova S, et al. Malignant triton tumour exhibits a complete expression pattern of nuclear retinoid and rexinoid receptor subtypes. Gen Physiol Biophys 2009;28:425-7. [Crossref] [PubMed]
- Sönmez K, Türkyilmaz Z, Karabulut R, et al. A triton tumor mimicking sacrococcygeal teratoma. J Pediatr Surg 2009;44:e5-8. [Crossref] [PubMed]
- James G, Crocker M, King A, et al. Malignant triton tumors of the spine. J Neurosurg Spine 2008;8:567-73. [Crossref] [PubMed]
- Cano JR, Algar FJ, Alvarez A, et al. Triton tumor of the left sympathetic nerve. Interactive CardioVascular and Thoracic Surgery 2006;5:790. [Crossref] [PubMed]
- Sierota D, Stefanowicz J, Wierzba J, et al. Neurofibromatosis type 1 in children. Experiences of the gdansk paediatric oncohaematology centre. Preliminary results. Med Wieku Rozwoj 2007;11:307-12. [PubMed]
- Ferrari A, Bisogno G, Macaluso A, et al. Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer 2007;109:1406-12. [Crossref] [PubMed]
- Chao MM, Levine JE, Ruiz RE, et al. Malignant triton tumor in a patient with li-fraumeni syndrome and a novel tp53 mutation. Pediatr Blood Cancer 2007;49:1000-4. [Crossref] [PubMed]
- Bień E, Stachowicz-Stencel T, Polczynska K, et al. Therapeutic difficulties in soft tissue sarcoma occurring in children with neurofibromatosis type 1 - own observations. Med Wieku Rozwoj 2008;12:477-84. [PubMed]
- Bien E, Stachowicz-Stencel T, Sierota D, et al. Sarcomas in children with neurofibromatosis type 1-poor prognosis despite aggressive combined therapy in four patients treated in a single oncological institution. Childs Nerv Syst 2007;23:1147-53. [Crossref] [PubMed]
- Masson P. Recklinghausen's neurofibrornatosis. Sensory neuromas and motor neuromas. Libman anniversary volumes 2. New York: International Press, 1932.
- Locatelli P. Formation de Membres Surnumeraires. CR Assoc des Anotomistes, 20 e reunion Turin, 1925:279-82.
- Woodruff JM, Chernik NL, Smith MC, et al. Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant "triton" tumors). Cancer 1973;32:426-39. [Crossref] [PubMed]
- Nikitin AYu, Lennartz K, Pozharisski KM, et al. Rat model of the human "triton" tumor: Direct genetic evidence for the myogenic differentiation capacity of schwannoma cells using the mutant neu gene as a cell lineage marker. Differentiation 1991;48:33-42. [Crossref] [PubMed]
- Holimon JL, Rosenblum WI. "Gangliorhabdomyosarcoma": A tumor of ectomesenchyme. Case report. J Neurosurg 1971;34:417-22. [Crossref] [PubMed]
- Naka A, Matsumoto S, Shirai T, et al. Ganglioneuroblastoma associated with malignant mesenchymoma. Cancer 1975;36:1050-6. [Crossref] [PubMed]
- Nelms BL, Labosky PA. Transcriptional control of neural crest development. San Rafael (CA): Morgan & Claypool Life Sciences; 2010.
- DiMaio SM, Mackay B, Smith JL Jr, et al. Neurosarcomatous transformation in malignant melanoma: An ultrastructural study. Cancer 1982;50:2345-54. [Crossref] [PubMed]
- Karcioglu Z, Someren A, Mathes SJ. Ectomesenchymoma. A malignant tumor of migratory neural crest (ectomesenchyme) remnants showing ganglionic, schwannian, melanocytic and rhabdomyoblastic differentiation. Cancer 1977;39:2486-96. [Crossref] [PubMed]
- McComb EN, McComb RD, DeBoer JM, et al. Cytogenetic analysis of a malignant triton tumor and a malignant peripheral nerve sheath tumor and a review of the literature. Cancer Genet Cytogenet 1996;91:8-12. [Crossref] [PubMed]
- Jhanwar SC, Chen Q, Li FP, et al. Cytogenetic analysis of soft tissue sarcomas. Recurrent chromosome abnormalities in malignant peripheral nerve sheath tumors (mpnst). Cancer Genet Cytogenet 1994;78:138-44. [Crossref] [PubMed]
- Rey JA, Bello MJ, Kusak ME, et al. Involvement of 22q12 in a neurofibrosarcoma in neurofibromatosis type 1. Cancer Genet Cytogenet 1993;66:28-32. [Crossref] [PubMed]
- Decker HJ, Cannizzaro LA, Mendez MJ, et al. Chromosomes 17 and 22 involved in marker formation in neurofibrosarcoma in von recklinghausen disease. A cytogenetic and in situ hybridization study. Hum Genet 1990;85:337-42. [Crossref] [PubMed]
- Haddadin MH, Hawkins AL, Long P, et al. Cytogenetic study of malignant triton tumor: A case report. Cancer Genet Cytogenet 2003;144:100-5. [Crossref] [PubMed]
- Travis JA, Sandberg AA, Neff JR, et al. Cytogenetic findings in malignant triton tumor. Genes Chromosomes Cancer 1994;9:1-7. [Crossref] [PubMed]
- Reynolds JE, Fletcher JA, Lytle CH, et al. Molecular characterization of a 17q11.2 translocation in a malignant schwannoma cell line. Hum Genet 1992;90:450-6. [Crossref] [PubMed]
- Kawai A, Noguchi M, Beppu Y, et al. Nuclear immunoreaction of p53 protein in soft tissue sarcomas. A possible prognostic factor. Cancer 1994;73:2499-505. [Crossref] [PubMed]
- Strauss BL, Gutmann DH, Dehner LP, et al. Molecular analysis of malignant triton tumors. Hum Pathol 1999;30:984-8. [Crossref] [PubMed]
- Bridge RS Jr, Bridge JA, Neff JR, et al. Recurrent chromosomal imbalances and structurally abnormal breakpoints within complex karyotypes of malignant peripheral nerve sheath tumour and malignant triton tumour: A cytogenetic and molecular cytogenetic study. J Clin Pathol 2004;57:1172-8. [Crossref] [PubMed]
- Magrini E, Pragliola A, Fantasia D, et al. Acquisition of i(8q) as an early event in malignant triton tumors. Cancer Genet Cytogenet 2004;154:150-5. [Crossref] [PubMed]
- James JA, Bali NS, Sloan P, Shanks JH. Low-grade malignant triton tumor of the oral cavity: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:699-704. [Crossref] [PubMed]
- Aldlyami E, Dramis A, Grimer RJ, et al. Malignant triton tumour of the thigh--a retrospective analysis of nine cases. Eur J Surg Oncol 2006;32:808-10. [Crossref] [PubMed]
- Radovanovic D, Vukotic-Maletic V, Stojanovic D, et al. Retroperitoneal "triton" tumor. Hepatogastroenterology 2008;55:527-30. [PubMed]


