
Successful umbilical cord blood transplantation in children with leukocyte adhesion deficiency type I
Introduction
Leukocyte adhesion deficiency type I (LAD-I) is a rare primary immunodeficiency disease with an approximate incidence of 1/1,000,000 (1). LAD-I is an autosomal recessive inherited condition. Mutations in the integrin beta chain-2 gene (ITGB2) leads to defective CD18 molecules on the surface of leukocytes, affecting leukocyte adhesion and their ability to migrate to inflammatory sites properly (2). Clinical characteristics of LAD-I patients include delayed separation of the umbilical cord, recurrent infections, and marked leukocytosis (3,4). Patients with CD18-expressing leukocytes at levels lower than 2% are defined as having severe LAD-I. Most patients with severe LAD-I die early in life due to infections. When patients with severe LAD-I do not undergo hemopoietic stem cell transplantation, death is 75% by the age of 2 years. Patients with CD18-expressing leukocytes at levels of 2–30% are defined as having moderate LAD-I. Most patients with moderate LAD-I survive childhood, but often experience recurrent infections and usually have poor long-term outcomes (5). However, it has been reported (6) that the inhibition of interleukin-23 and interleukin-17 may play a role in the management of patients with moderate LAD-I. Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative method for patients with LAD-I, especially for severe type. The severe type of LAD-I is fatal by the age of 2 years without allo-HSCT (7). Previous publications have indicated that allo-HSCT donors are usually HLA-identical siblings or matched unrelated donors (MUDs) with umbilical cord blood transplantations (UCBT) being rare (8,9). Here, we report the outcomes for five consecutive patients with LAD-I who underwent UCBT.
Methods
All five patients underwent UCBT using cells from unrelated donors. None of the patients had HLA-identical related donors or suitable unrelated donors in the China Marrow Donor Program. The five umbilical cord blood units were obtained from cord blood banks in China that had been approved by the Chinese National Ministry of Health. The median age and body weight at the time of UCBT was nine months (range, 8 to 132 months) and 8.0 kg (range, 5.0 to 24.3 kg), respectively.
Patients
The Ethics Committee approved this study of Children’s Hospital of Fudan University. Written informed consent was obtained from the guardians of all patients before treatments. A total of five consecutive patients with LAD-I (two boys and three girls) underwent UCBT at our medical center from September 2016 to September 2018. The diagnoses of LAD-I were based on typical clinical manifestations, CD18 expression levels, and gene sequencing (10,11). The expression of CD18 was detected using flow cytometry, and gene mutations were found by next-generation sequencing and Sanger sequencing (12).
HLA compatibility and origin of umbilical cord blood
HLA identity was based on A, B, Cw, DRB1, and DQ, which were determined using high-resolution molecular techniques. Units of umbilical cord blood were selected according to cellularity and compatibility. Cord blood transplantations for the patients were dosed at more than 3×107 total nucleated cells (TNC)/kg, and CD34+ cells were dosed at 1×105 cells/kg. HLA identity greater than 7/10 was used. One of the five cord blood units was matched at an HLA identity of 10/10, three units at 9/10, and the other unit at 8/10. The data collected included clinical characteristics and laboratory data, such as basic patient information, infections, white blood cell counts, abnormal genes, CD18 expression on the surface of leukocyte, conditioning regimens, HLA matching, CD34-positive cell dose, engraftment, complications, immune reconstitution, and outcomes. The main transplant characteristics are shown in Table 1.
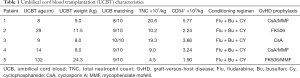
Full table
Conditioning regimen
All five patients received the same conditioning regimen. The regimen included busulfan (Bu) at a dose of 1–1.2 mg/kg given intravenously (i.v.) every 6 hours for 4 days (total dose, 16–19.2 mg/kg), fludarabine (Flu) at 35 mg/m2/d or 1.4 mg/kg/d by i.v. for 5 days (total dose, 175 mg/m2 or 7 mg/kg), and cyclophosphamide (CY) at 50 mg/kg/d by i.v. for 2 days (total dose, 100 mg/kg). Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporin A (CsA), mycophenolate mofetil (MMF), or tacrolimus.
Infection prophylaxis
All the patients were kept in single laminar airflow rooms until their absolute neutrophil count (ANC) exceeded 0.5×109 cells/L for three consecutive days. All patients received cytomegalovirus prophylaxis of ganciclovir (10 mg/kg/d) from the first day of conditioning until day −1, herpes virus prophylaxis of acyclovir (750 mg/m2/d) from day 0 until day +270, candida prophylaxis of micafungin (4–6 mg/kg/d) from the first day of conditioning until neutrophils engraftment, Aspergillus prophylaxis of voriconazole (10 mg/kg/d) from neutrophil engraftment until day +180, and Pneumocystis carinii prophylaxis of compound sulfamethoxazole (25 mg/kg/d) twice a week from the time of neutrophil engraftment until 6 months after immune reconstitution. Patients also received iv treatments of immunoglobulin (500 mg/kg/dose) every 2 weeks starting on day +1 post-UCBT and continuing until B-lymphocyte levels recovered.
Chimerism studies and CD18 expression
Chimerism studies were performed using DNA isolated from peripheral blood samples collected on day +14, +30, +60, +100, +180, +270, +365. The DNA was analyzed by polymerase chain reaction (PCR) for short tandem repeats (STR). Complete chimerism was defined as the presence of more than 95% donor-derived cells in whole blood, while mixed chimerism was defined as the presence of more than 5% host-derived cells. High-level mixed chimerism was defined as 50–95% donor chimerism. Less than 5% of donor chimerism was considered engraftment failure. Primary disease assessment was performed on day +60 using flow cytometric to screen for CD18-positive leukocytes in peripheral blood.
Results
Patients’ characteristics
The median age of the five patients at the time of diagnosis was 13 months (range, 4 to 120 months). All five patients had typical granulocytosis. One patient had severe LAD-I with the level of CD18-expressing neutrophils being 0.8%. The other four patients had moderate LAD-I with the levels of CD18-expressing neutrophils being 2.4–3.6%. ITGB2 mutations were observed in all the patients. Pre-transplant complications included five cases of pneumonia, three cases of omphalitis, three cases of skin and soft tissue infections, two cases of sepsis, and two cases of intestinal infection, as well as other infections. The specific conditioning regimens and prophylaxis of GvHD for the five patients are shown in Table 1. The main patient characteristics are summarized in Table 2.
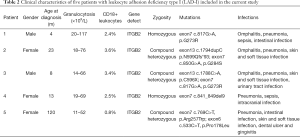
Full table
Umbilical cord blood cell infusion and engraftment
In the five patients, the median TNC dose infused was 10.2×107/kg (range, 4.5×107 to 20.6×107/kg) and CD34+ cell dose was 3.2×105/kg (range, 1.9×105 to 5.7×105/kg). Neutrophil counts ≥0.5×109 cells/L for three consecutive days, and platelet count ≥20×109 cells/L for seven consecutive days without transfusion were considered successful engraftments. All the patients with LAD-I engrafted. The median number of days of neutrophil engraftment was 20 days (range, 13 to 28 days). The median number of days of platelet engraftment was 36 days (range, 32 to 56 days). All patients maintained greater than 95% complete donor chimerism (CDC). Four of the patients achieved CDC by day +14 post-UCBT, and the other patient achieved CDC at 4 months post-UCBT. The expression of CD18 was evaluated on day +60, with all results being normal. The reconstitution of lymphocyte subtypes is shown in Figures 1-5.
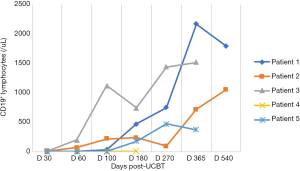
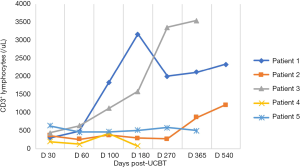
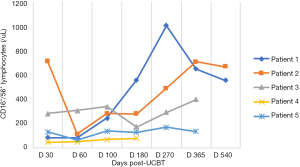
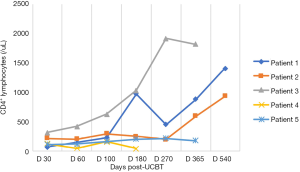
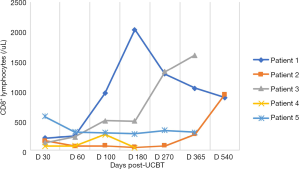
GvHD
Four of the five patients developed grade II–IV acute GvHD (aGvHD). One patient had acute grade IV GvHD (stage 3 skin and stage 4 liver), which was controlled with CsA, MMF, and high-dose methylprednisolone pulse therapy (10 mg/kg for 3 days). One patient with grade II skin aGvHD improved following treatment with oral methylprednisolone. For one patient with grade III GvHD (stage 3 skin and stage 3 gut), the condition was controlled by infusion of mesenchymal stem cells (MSC) and improved following treatment with budesonide. Another patient with grade II skin aGvHD was treated with oral methylprednisolone and MSC infusion.
Complications and survival
The median follow-up time was 19 months (range, 8 to 38 months), with four of the five patients surviving. Two patients developed CMV viremias and were treated with ganciclovir or valganciclovir. Two patients suffered from sepsis (Escherichia coli and Pseudomonas aeruginosa, respectively) and were treated with appropriate antibiotic treatments. Two patients developed bronchiolitis obliterans after UCBT. One of the cases was controlled with methylprednisolone, but the other patient died of respiratory failure eight months after UCBT. Full details regarding complications and survival are provided in Table 3.

Full table
Discussion
Although LAD-I is a rare primary immunodeficiency disorder from 1975 to 2017 there were more than 320 recorded cases worldwide (9), with only a few reported cases in China (13-16). The pathogenesis of LAD-I is associated with ITGB2 mutations that cause defects in CD18 of the β2 integrin family, resulting in leukocyte adhesion dysfunction and the inability of the cells to migrate to inflammatory sites (2). Repeated bacterial infections with marked leukocytosis characterized clinical symptoms. The first symptoms are usually omphalitis and delayed umbilical cord separation. Other infections may include skin mucosa and soft tissue infection, otitis media, respiratory infections, and sepsis, among others (17).
Five LAD-I diagnosed patients were admitted to our center based on gene sequencing analyzes and included in the current study. Four of the patients had experienced recurrent infections and leukocytosis after birth. The onset symptom of the other patient was a dental ulcer and gingivitis at the age of one year.
HSCT is the primary curative approach to treat patients with LAD-I. The patients in the current study received UCBT, and four of the five achieved disease-free survival. Outcomes are available in the literature for 101 of 125 patients with LAD-I that received allo-HSCT from 1981 to 2017. The overall survival rate of these patients was approximately 75% (9). Most of the patients were treated with matched sibling donor (MSD) or MUD transplantation, which accounted for 35.6% and 24.7%, respectively. The success rate of transplantation was 83.3% for MSD and 76% for MUD. The transplantation-related mortality rates were 11% for MSD, and 24% for MUD and engraftment failure rates were 5.6% and 0%, respectively. Among these patients, six underwent UCBT with four achieving phenotypic correction and two suffering engraftment failure; one of the patients experiencing engraftment failure died (18,19). Al-Dhekri (20) reported on 11 patients with LAD-I that were treated by HSCT, including three patients, were treated with UCBT. One of the patients achieved complete chimerism after the first UCBT, one had mixed chimerism, and the third exhibited complete chimerism after a second UCBT. The event-free survival rate was 100%. The nine cases reported in the literature of patients with LAD-I that received UCBT are detailed in Table 4. The overall success rate of these patients is 77.8% with no transplant-related deaths. The engraftment failure rate is 22.2%. Compared with MSD or MUD as donor sources, the engraftment failure rate of UCBT is higher but the transplantation-related mortality rate of it was lower.

Full table
Hamidieh (19) reported engraftment failure for a patient with LAD-I that was treated with UCBT and had received reduced-intensity conditioning (RIC) as a myeloablative conditioning (MAC) regimen consisting of Flu + melphalan (Mel) + anti-thymocyte globulin (ATG). Another patient received Bu + etoposide (VP16) as MAC before UCBT, which resulted in mixed chimerism. Three other patients were treated for MAC using Bu + CY + ATG, and each achieved complete chimerism without severe GvHD. Al-Dhekri (20) reported on three patients treated for MAC with Bu + CY + ATG, of which two were successfully engrafted with one case resulting in mixed chimerism. The patient who suffered engraftment failure received a secondary UCBT after a MAC regimen of CY + ATG + total body irradiation (TBI) and was successfully engrafted. Therefore, when UCBT is considered in the treatment of patients with LAD-I, if the patient’s condition is tolerable, it is advisable to include a MAC regimen to facilitate the engraftment of umbilical cord blood stem cells.
For our patients, we adopted a MAC regimen consisting of BU + FLU + CY. Due to the risk of viral infections and delayed immune reconstitution following UCBT, which can be fatal, our conditioning regimen did not include ATG. All five of the patients with LAD-I treated at our center with UCBT after MAC achieved engraftment, CDC, and correction of both immunophenotype and genotype. Four of the patients survived and achieved complete remission. One of the patients achieved CDC at 4 months post-UCBT.
Due to the obvious increase of leukocyte numbers and excessive activity of phagocytes in patients with LAD-I, mixed chimerism may occur after transplantation, especially in patients that receive an RIC regimen. It is reported in the literature for patients with LAD-I that have stable mixed chimerism after HSCT that they may survive asymptomatically for an extended time (18,19). This suggests that mixed chimerism may be enough to prevent the occurrence of LAD-I-related symptoms. Animal studies have also shown that low levels of functional CD18-expressing white blood cells can prevent the occurrence of disease and even permanently reverse the state of disease (21,22). However, for patients with mixed chimerism, it is necessary to regularly follow-up to avoid graft rejection.
GvHD is the main complication following transplantation, and severe GvHD can affect patient prognosis. Compared with bone marrow-derived stem cells, cord blood stem cells are more primitive and less immunogenic. Therefore, theoretically, the incidence and severity of GvHD should be low. This is supported by Qasim (18), who reported that for 12 cases of bone marrow-derived stem cell transplantation (except those that received MSD), three developed grade II–III aGvHD and two developed grade III/IV aGvHD, while only one case developed grade I-II aGvHD among five cases of patients that received transplants of umbilical cord blood stem cell.
Furthermore, Al-Dhekri (20) reported three cases of umbilical cord blood stem cell transplantation in children without the development of GvHD. Our center adopted a MAC regimen without ATG, and four of the five patients developed grade II/III aGvHD after transplantation. Compared with these earlier reports, our incidence and severity of aGvHD were higher. However, after treatment with glucocorticoid, calcineurin inhibitors, and MSC infusion, their conditions improved.
MSDs are the best choice for allo-HSCT, but not every patient is lucky enough to have an MSD. Fortunately, umbilical cord blood stem cells are an alternative and are relatively easy to obtain. Furthermore, umbilical cord blood has advantages of low immunogenicity, relatively low incidence of GvHD, and a relatively low possibility of virus transmission. Umbilical cord blood stem cells are also more suitable for young children with low body weight. UCBT was successfully performed in our center for five children with LAD-I. Our results confirmed the feasibility and effectiveness of UCBT for treating patients with LAD-I.
In conclusion, umbilical cord blood stem cell transplantation is an effective method to treat patients with LAD-I. Children with LAD-I, especially those with a severe type, should receive allo-HSCT as soon as possible to attain better long-term survival. The limitation of this study is a limited number of patients in a single Chinese center. We need multi-center collaborations to enlarge sample and furtherly study in the efficacy of UCBT in this rare disease in the future.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Ethics Committee approved this study of Children’s Hospital of Fudan University. Written informed consent was obtained from the guardians of all patients before treatments.
References
- Harris ES, Weyrich AS, Zimmerman GA. Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr Opin Hematol 2013;20:16-25. [PubMed]
- Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol 2013;55:49-58. [Crossref] [PubMed]
- Chang IJ, He M, Lam CT. Congenital disorders of glycosylation. Ann Transl Med 2018;6:477. [Crossref] [PubMed]
- van de Vijver E, Van den Berg TK, Kuijpers TW. Leukocyte adhesion deficiencies. Hematol Oncol Clin North Am 2013;27:101-16. [Crossref] [PubMed]
- Fischer A, Lisowskagrospierre B, Anderson DC, et al. Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic Rev 1988;1:39-54. [PubMed]
- Moutsopoulos NM, Zerbe CS, Wild T, et al. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med 2017;376:1141-6. [Crossref] [PubMed]
- Simpson AM, Chen K, Bohnsack JF, et al. Pyoderma gangrenosum-like wounds in leukocyte adhesion deficiency: case report and review of literature. Plast Reconstr Surg Glob Open 2018;6:e1886. [Crossref] [PubMed]
- Thomas C, Le Deist F, Cavazzana-Calvo M, et al. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood 1995;86:1629-35. [Crossref] [PubMed]
- Almarza Novoa E, Kasbekar S, Thrasher AJ, et al. Leukocyte adhesion deficiency-I: a comprehensive review of all published cases. J Allergy Clin Immunol Pract 2018;6:1418-20.e10. [Crossref] [PubMed]
- Roos D, Meischl C, Boer MD, et al. Genetic analysis of patients with leukocyte adhesion deficiency: genomic sequencing reveals otherwise undetectable mutations. Exp Hematol 2002;30:252-61. [Crossref] [PubMed]
- van de Vijver E, Maddalena A. Hematologically important mutations: leukocyte adhesion deficiency (first update). Blood Cells Mol Dis 2012;48:53-61. [Crossref] [PubMed]
- Sun B, Chen Q, Dong X, et al. Report of a Chinese Cohort with Leukocyte Adhesion Deficiency-I and Four Novel MutationsJ. J Clin Immunol 2019;39:309-15. [Crossref] [PubMed]
- Liu JR, Zhao SY, Jiang ZF. Clinical analysis of a Chinese child with leukocyte adhesion deficiency type 1. Zhonghua Er Ke Za Zhi 2013;51:531-4. [PubMed]
- Wang T, Jiang LP, Gao H, et al. Clinical and molecular features of one case of leukocyte adhesion deficiencytype-1. Immunol J 2017;33:697-702.
- Lin Y, Zheng HY, Xian YY, et al. Novel mutations of ITGB2 induced leukocyte adhesion defect type 1. Zhonghua Er Ke Za Zhi 2018;56:617-22. [PubMed]
- A novel mutation of the ITGB2 gene in a Chinese Zhuang minority patient with leukocyte adhesion deficiency type 1 and glucose-6-phosphate dehydrogenase deficiency. Gene 2019;715:144027. [Crossref] [PubMed]
- Pereira JL, Gomes M, Teixeira AL, et al. Potential and importance of metalloproteinases and interleukins in inflammation and metastasization in non-small cell lung cancer. Transl Cancer Res 2018;7:782-95. [Crossref]
- Qasim W, Cavazzana-Calvo M, Davies EG, et al. Allogeneic hematopoietic stem-cell transplantation for leukocyte adhesion deficiency. Pediatrics 2009;123:836-40. [Crossref] [PubMed]
- Hamidieh AA, Pourpak Z, Hosseinzadeh M, et al. Reduced-intensity conditioning and hematopoietic SCT for pediatric patients with LAD-1: clinical efficacy and importance of chimaerism. Bone Marrow Transplant 2012;47:646-50. [Crossref] [PubMed]
- Al-Dhekri H, Al-Mousa H, Ayas M, et al. Allogeneic hematopoietic stem cell transplantation in leukocyte adhesion deficiency type 1: a single center experience. Biol Blood Marrow Transplant 2011;17:1245-9. [Crossref] [PubMed]
- Creevy KE, Bauer TR Jr, Tuschong LM, et al. Mixed chimeric hematopoietic stem cell transplant reverses the disease phenotype in canine leukocyte adhesion deficiency. Vet Immunol Immunopathol 2003;95:113-21. [Crossref] [PubMed]
- Bauer TR Jr, Allen JM, Hai M, et al. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat Med 2008;14:93-7. [Crossref] [PubMed]


